Background – To evaluate efficacy and morbidity prospectively in a contemporary multi-institutional salvage radical prostatectomy (SRP) series.
Methods – Forty-one men were enrolled between 1997 and 2006, who suffered biopsy-proven recurrent prostate cancer (CaP) after receiving ≥ 60c Gy radiation as primary treatment for cT1–2NXM0 disease. Surgical morbidity, quality of life, biochemical progression-free survival (BPFS) and overall survival (OS) were evaluated.
Results – Twenty-four men had undergone external beam radiotherapy, 11 brachytherapy, and six both. Median time between radiation and SRP was 64 months. Median age at SRP was 64 years. Pathologic staging revealed 44% pT2, 54% pT3, and 3% pT4. Surgical margins were positive in 17 and 88% were pN0. Twenty-two percent required intraoperative blood transfusion. Three rectal and one obturator nerve injuries occurred. Seventeen of 38 evaluable patients (45%) had urinary incontinence ( ≥ 3 pads/day) prior to SRP; 88% reported urinary incontinence at 6 months, 85% at 12 months, 63% at 24 months after SRP. Furthermore, 37% of men reported impotence prior to SRP; 78% reported impotence at 6 months, 82% at 12 months, and 44% at 24 months after SRP. The 2-, 5- and 10-year BPFS rates were 51, 39, and 33% respectively; the 2-, 5- and 10-year OS rates were 100, 89, and 52%, respectively, at median follow-up 91 months.
Conclusions – Modern surgical techniques continue to be associated with significant peri-operative complication rates. Nevertheless, SRP may benefit carefully selected patients through durable oncologic control.
Introduction
Twenty-five to 35% of men with clinically localized prostate cancer (CaP) receive radiation therapy as definitive treatment1,2. Despite improvements in patient selection, delivery techniques, and use of concomitant systemic therapy, some men recur the disease. Five-year biochemical progression-free survival (BPFS) rates after radiotherapy range 75–85% for low risk disease to 30–70% for high-risk disease3-6. Biopsy-documented local recurrence 2–10 years after radiation can reach 35% in contemporary and 90% in historical series7-9. CaP local recurrences after radiotherapy may increase risk of developing local urinary symptoms and the potential for subsequent metastasis10.11. One therapeutic approach is salvage radical prostatectomy (SRP). SRP has been perceived poorly due to high coincidence of systemic disease and high complication rates reported in early surgical series12-17. However, the growing number of CaP patients receiving radiation as primary therapy18 has led to re-emergence of SRP for radiation-recurrent or resistant disease19, particularly given the potential for improved outcomes compared to historical series, given earlier diagnosis in the PSA era, better initial stratification of patients and improvements in SRP technique19-21.
Cancer and Leukemia Group B (CALGB) 9687 was a prospective, multi-institutional study designed to evaluate the feasibility of SRP as demonstrated by surgical morbidity, quality of life, pathologic outcomes, BPFS and overall survival (OS) in patients with local recurrence after primary radiotherapy. CALGB 9687 was the first CALGB surgical trial to include quality assurance data that were collected prospectively and evaluated annually. To our knowledge this was the first multi-center prospective trial to evaluate SRP in a cooperative group setting. We hypothesized that SRP would be feasible with respect to morbidity and quality of life outcomes in a multi-institutional setting.
Materials and methods
Institutional Review Board approval from each participating site and written protocol-specific informed patient consent were required for study participation. Patient registration and data collection were managed by CALGB (Alliance for Clinical Trials in Oncology, Statistical Center) [CALGB 9687 ClinicalTrials.gov NCT00002938].
Eligibility
Protocol design
After study enrollment, patients underwent open SRP. A pelvic lymph node dissection was required but extent was not specified. Frozen section evaluation of lymph nodes was performed at the time of SRP and SRP was aborted for lymph node metastasis. SRP included complete removal of the prostate and seminal vesicles. Intra- and 30-day perioperative SRP morbidities were collected and evaluated. Major complications were defined as rectal injuries, urinary incontinence (defined as ≥ 3 pads/day), transfusion of nonautologous blood, deep vein thrombosis, pulmonary embolus, myocardial infarction, stroke, life-threatening complication, or death. Minor complications were defined as wound infection, prolonged ileus, urinary tract infection, bladder neck contracture, lymphedema, or stress urinary incontinence ( < 3 pads/day). Quality of life assessments consisted of FACT-P and a specifically-designed Bladder, Bowel, and Sexual Functioning Inventory Form (CALGB Form C-466). Questionnaires were administered by treating physicians and/or clinical research nurses prior to SRP and 3, 6, 9, 12, 18, and 24 months after SRP. Clinical complete response was defined as disappearance of all measurable disease, signs, symptoms and biochemical changes related to CaP. Biochemical progression was defined as an increase in serum PSA level to > 0.2 ng/ml on two successive measurements at least 2 weeks apart.
Statistical design and data analysis
The primary endpoint was BPFS, defined as biochemical progression or death from CaP, whichever occurred first. The target sample size was 40 patients. Assuming an exponential distribution for BPFS, 2-year BPFS rate 81%, accrual rate 20 patients per year over 2 years, and 2 years of post-accrual follow-up, with 40 patients the expected lower bound on a one-sided 95% confidence interval (C1) for 2- year BPFS progression rate was 69%. The Kaplan-Meier product-limit approach was used to estimate BPFS and OS distributions. Data quality was ensured by careful review of data by the CALGB Statistical Center staff and the study chair. Members of the Data Audit Committee visited all participating institutions at least every 3 years to review source documents. Auditors verified compliance with federal regulations and protocol requirements, which included those pertaining to eligibility, treatment, adverse events, tumor response and outcome in a sample of protocols at each institution. Such on-site review of medical records was performed for a subgroup of 16 of the 49 patients (33%) on study.
Results
Forty-nine patients with radiation-resistant or recurrent CaP were enrolled between May 1997 and March 2006. Two patients were found later to be ineligible for analysis and six did not undergo SRP. Of these six patients, two operations were canceled for reasons unrelated to the study, three patients were found to have lymph node metastases at the time of operation so SRP was aborted and one patient was deemed unresectable due to body habitus and anatomic characteristics unique to that patient’s prostate gland. Thus, 41 patients were eligible for analysis with median time to follow-up 91 months (95% CI = 63–104 months). SRP was performed at a total of five institutions by a total of eight surgeons who performed from 1 to 16 cases each.
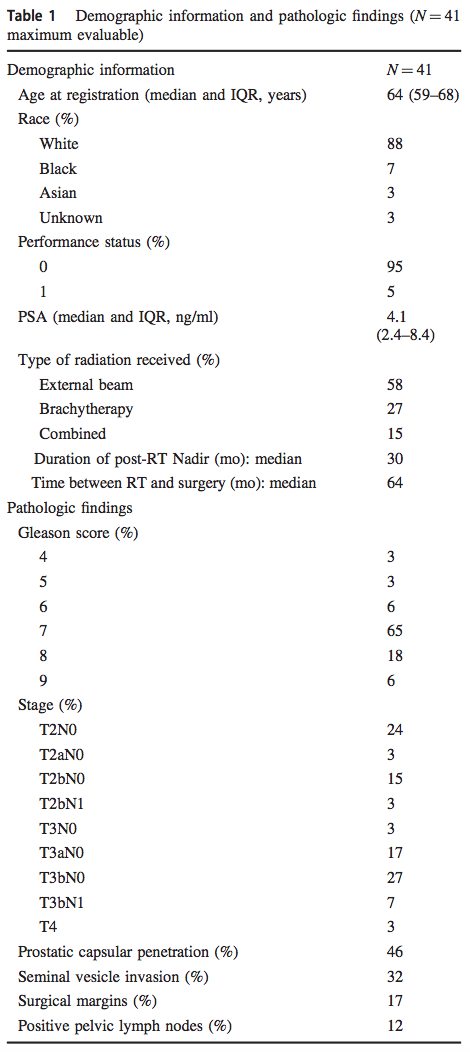
Demographics were summarized in Table 1. Median age at SRP was 64. Primary radiotherapy included external beam radiotherapy22, brachytherapy11or external beam and brachytherapy6. Doses of external beam radiation ranged 65–81 Gy and brachytherapy used iodine or palladium seeds. Median duration of post-radiotherapy PSA nadir was 30 months (IQR 21–46 months) and median time between radiation and SRP was 64 months (IQR 35–86 months). Median PSA immediately prior to SRP was 4.1 ng/ml (IQR 2.4–8.4).
Pre-radiation treatment data from 34 of 41 patients were presented in Table 2. Median PSA was 5.4 ng/ml. One patient had Gleason score 4, 2 had Gleason score 5, 12 had Gleason score 6, 16 had Gleason score 7 and 3 had Gleason score 8 disease. All patients were deemed to have localized disease and felt appropriate for primary radiation therapy, but pre-radiation treatment clinical stages were available for only 26 patients: 10 were cT1c, 14 were cT2 and 2 were cT3.
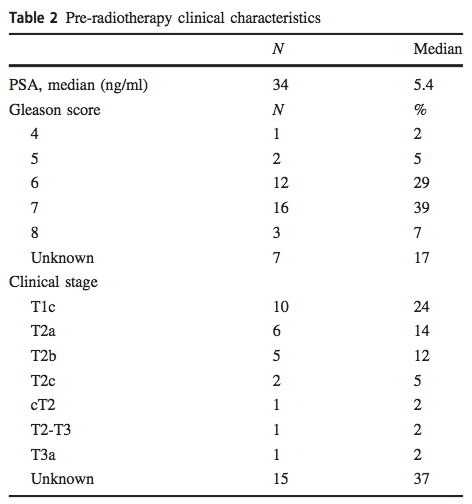
Pathologic findings after SRP were summarized in Table 1. Pathologic stage was pT2 in 44%, pT3 in 54%, and pT4 in 3%. All patients underwent pelvic lymphadenectomy. Median number of nodes removed was 7 (IQR 4–10). Five patients (12%) were pN1. Seven patients (17%) had positive surgical margins.
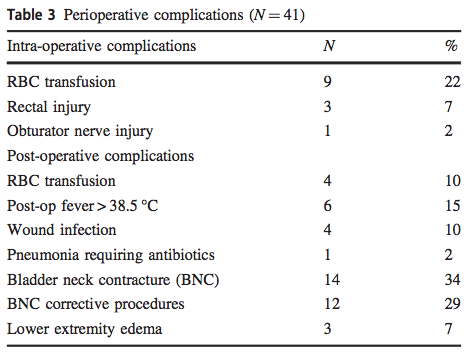
Perioperative outcomes were presented in Table 3. Median operative time was 213 minutes (range 138–532). Blood transfusions were required for 22% of patients intraoperatively and 10% of patients post-operatively. No perioperative deaths occurred. Three rectal injuries and one obturator nerve injury occurred. Four patients suffered anastomotic urinary leaks. Lower extremity edema occurred in three patients and one patient suffered pulmonary embolus. Two lymphoceles required drainage. Patients had four wound infections (one at the harvest site of a sural nerve graft) and one wound dehiscence. Bladder neck contractures required corrective procedures in 34%, which included eight direct vision internal urethrotomies (DVIU), four dilatations and three transurethral resections of the bladder neck (TURBN). Secondary operations included artificial urinary sphincter (AUS) placement in four patients.
Questionnaires revealed 17 of 38 patients (45%) had baseline incontinence ( ≥ 3 pads/day) prior to SRP. Not all questionnaires were completed and submitted for all patients at all time points. Thirty-five of 40 patients (88%) reported urinary incontinence at 6 months, 29 of 34 (85%) at 12 months, 20 of 32 (63%) at 24 months, and 10 of 24 (42%) at 36 months after SRP.
Prior to SRP, 12 of 38 (32%) of patients reported erectile dysfunction. Erectile dysfunction was reported frequently after SRP; 90% (35 of 40 patients) had erectile dysfunction at 3 months after SRP. At 6 months, 31 of 40 (78%) reported erectile dysfunction that improved to 6 of 24 (25%) 3 years after SRP.
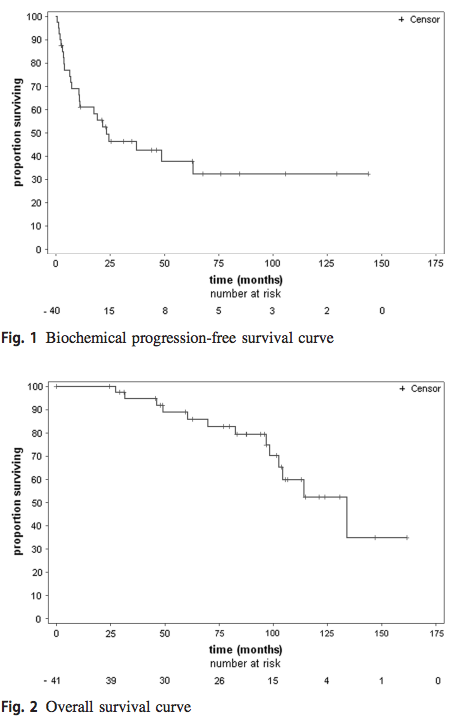
Median BPFS was 24.4 months (95% CI: 10.9-not reached). The 2-, 5- and 10-year BPFS rates were 51% (95% CI: 34–65%), 39% (95% CI: 2–55%) and 33% (95% CI: 17–51%), respectively (Fig. 1). Nine patients received androgen-deprivation therapy subsequent to biochemical recurrence; post-operative non-protocol therapies were not collected formerly. The median OS time was 134 months (95% CI: 98.5- not reached). The 2-, 5- and 10-year OS rates were 100%, 89% (95% CI: 73–96%), and 52% (95 CI: 29–71%), respectively (Fig. 2).
Discussion
SRP was associated with poor cancer control and significant morbidity when CALGB 9687 was initiated in 199712-17. The trial was designed to evaluate the feasibility of SRP in the PSA era using modern anatomic radical retropubic surgical techniques and improved staging and stratification techniques. However, CALGB 9687 was unique because it was also a prospective multi-institutional study, designed specifically with validated QOL parameters monitored dynamically, to determine whether the earlier retrospective reports (largely single institution chart reviews) could be verified and improved upon. Since the inception of CALGB 9687, results from several contemporary series have been published as retrospective reviews19, 22-25. These series have demonstrated improved progression-free and cancer-specific mortality with diminished rates of complications compared to historical controls12-17.
A major concern for any salvage therapy has been determining whether there is disease spread at the time of salvage treatment. In recent SRP series, organ-confined disease was found in < 50% of patients, seminal vesicle invasion ranged 25–63% and pelvic lymph node involvement ranged 16–50%19, 26, 27. In CALGB 9687, 44% of patients had organ-confined disease; of those with locally advanced disease more than half had seminal vesicle involvement (pT3b) (34% of all men). Seven patients (17%) had positive surgical margins. In a multi-institutional series of 404 patients, Chade et al. reported a 25% positive margin rate19. In two single institutional experiences, Sanderson et al.27 and Heidenreich et al.26 reported positive margin rates of 36 and 13%, respectively. Our results for margin rates after SRP are consistent with these contemporary series. The low positive surgical margin rates may reflect improvements in patient selection and surgical experience. The low rate of nodal involvement (12% of patients who underwent SRP and 18% of enrolled and operated patients [three patients had positive lymph nodes and did not undergo SRP]) might be attributed to improved pre-operative stratification of men with CaP in the PSA era.
Radiation-induced vasculitis, fibrosis and tissue plane obliteration are factors that can cause SRP complications, such as rectal injuries, anastomotic strictures, and incontinence22. Increased experience in radical prostatectomy among the group of CALGB 9687 surgeons kept SRP intraoperative complication rates at a reasonable but increased level compared to radical prostatectomy without prior radiation therapy and consistent with recently published series23-25.
The perception that SRP results in high rates of incontinence and universal erectile dysfunction have been major impediments to wide-spread acceptance of SRP. Urinary incontinence remains common after SRP and occurs in 50–80% of patients23-25. Definitions and assessment methods differ, which makes comparison across studies difficult. In the present series, baseline incontinence rates prior to SRP were much higher (45% of patients reported significant leakage after radiation) than would be expected with contemporary radiotherapy and may reflect earlier and less sophisticated targeting technologies employed when many of the men were treated. These unexpectedly high rates of pre-prostatectomy leakage are reflected in high post-prostatectomy incontinence rates (85% at 12 months). However, the incontinence rate at 3 years post-SRP (42%) was comparable to that seen pre-operatively. Interestingly, there was a continuing trend towards improvement at the 24- and 36-month time points, 63% and 24% respectively. This is contrary to the assumption that SRP patients do not have the capability to improve urinary control after 1 year. Among all patients, 10% were bothered sufficiently by incontinence that they had artificial urinary sphincters placed. These incontinence rates should be accurate since CALGB 9687 was among the first surgical trials to use standardized and validated health-related quality of life outcome measurement tools. However, using three pads a day as cut-off for urinary incontinence may exclude some men who had bothersome leakage.
Sexual dysfunction after SRP is reported in the literature to approach 100%. In this series, 37% of patients reported some degree of erectile dysfunction prior to SRP. At 6 months, 78% reported erectile dysfunction, which increased to 82% at 12 months. However, 3 years after SRP, only six of 24 (25%) reported erectile dysfunction. Many of these men were using mechanical and injection therapies, were sexually active and denied impotence when queried.
Prior series reported 5-year BPFS after SRP from 48–70% with 10-year rates as low as 28–45%12-15, 19. In CALGB 9687, the 5 and 10-year BPFS rates 39% and 33%, respectively; the 5 and 10-year OS rates were 89% and 52%, respectively. Interpretation of this study and historic results may be skewed, as the non-protocol treatments were not formally collected and may artificially lower the biochemical recurrence.
CALGB 9687 was open for accrual for 9 years; 75% of enrollment occurred in the last 5 years. A majority of the patients were enrolled at a single institution. Both of these issues may be contributed to by lack of established referral patterns from radiation oncologists to urologists for radiation treatment failures. This short-coming was compounded by historical perceptions of SRP as a high-risk operation with questionable cancer control and traditionally poor abilities to identify which radiation failure patients may be optimal candidates for additional local therapy. Contemporary series have demonstrated better oncologic outcomes when SRP has been performed earlier23, 25. However, the median interval from radiation therapy to SRP in the current study was 64 months. As the use of radiation as a primary modality has increased, so has the application of salvage therapies22, 28-30. Many patients who initially chose radiation therapy as definitive therapy did so because of concerns for post-operative incontinence and impotence and these concerns are increased with SRP. These trends may be changing due to the significant numbers of men with rising PSAs after radiation and increased experience of urologists with radical prostatectomy.
Radiation therapy for CaP also has changed since the study opened, and most of the men in the study did not have IMRT or high dose radiation, which are now considered standard of care. In addition, 75% of the enrolled subjects with known pre-radiation clinical staging had palpable disease. As a result, the enrolled patients do not represent a current population of radiation patients and therefore the enrolled patients should be expected to have worse outcomes after both radiation and SRP. Since the initiation of the study, it has become clear that those patients who respond best to salvage treatment have lower PSA values, Management of recurrent prostate cancer after radiotherapy: long-term results from CALGB 9687. . . 313 lower grade disease pre-radiation, and a prolonged postradiation nadir [19, 26]. In this regard, the study patients compare favorably: the median pre-radiation therapy PSA was 5.5 ng/ml and the majority had pre-treatment Gleason score 7 or less. In addition, the median duration of postradiotherapy PSA nadir was 30 months and the median time between radiation and SRP was over 5 years.
Since this study’s creation, robotic-assisted prostatectomy has become the predominate surgical managment for treatment naïve prostate cancer. Accordingly, several institutions have published small robotic SRP series (n = 6–51) and related outcomes31-35. While all are retrospective, there is similar surgical outcomes with positve surgical margins ranging 13–31% and node positive disease 0–18%. As an aggregate, these reviews report lower rate of rectal injuries (0–3%), bladder neck contractures (7–17%), and major incontinence (7–77%), but with higher amount of anastamotic leaks (7–33%) and comparable erectile dysfunction rates (71–100%). This should be interpreted with caution as these reviews intermitently reported preoprertative quality of life measures, and used a variety of instruments to assess. Most importantly, while the oncologic outcomes appear promising with BPFS ranging from 57–82%, only two studies had a median follow-up > 24 months (Kaffenberger et al., Yuh et al.), and in this study our median follow-up was 91 months with the median biochemical failure happening at 24.4 months.
Cryosurgery is another potential adjuvant treatment for radiation resistent prostate cancer. In an analysis of the Cryo On-Line Data (COLD) registry data, Spiess et al. compared 183 patients without ADT that underwent salvage cryotherapy after primary radiation treatment36. With a median follow-up of 36 months, 72.7% had persistent PSA < 0.6 ng/ml and were continent based on physician reporting. In an updated analysis of the COLD database and with stricter inclusion criteria, Pisters et al. demonstrated SRP had superior BPFS at 5 years defined as 2x PSA increases above nadir (SRP 66% and Cryo 42%, p < 0.01). This significance only trended when analyzing SRP against cryo patients that had two freeze thaw cycles (SRP 66% and Cryo 47%, p = 0.13)37. The COLD database studies excluded more aggressive features (ex: Gleason 9 + , and cT3b) and relied on physcian reporting of functional outcomes, making comparisons to the present study difficult.
We recognize this study has some limitations. This cohort of men represents a younger population than the average age of failure after initial treatment of prostate cancer. This may be the result of the eligibility criteria selecting ECOG of 0–1, selection bias from the surgeons, referral patterns from radiation oncologist, or patients electing for more aggressive treatment. Also, the protocol stipulated that frozen sections be performed at time of surgery and SRP be abandoned if positive, and yet 12% of patients were still pN1. This highlights the discordance between frozen section evaluation of grossly positive nodes and the micrometastic positive nodes seen after templated lymph node dissection38. In addition, aborting radical prostatectomy in treatment naïve patients is no longer the standard of care and completion prostatectomy may in fact benefit SRP. On a related note, the cohort was too small for post-hoc multivariable analysis to determine who was at greatest risk of treatment failure. Nerve sparing technique was not stipulated within the protocol and therefore the decision was left to the surgeon, which could have influenced our quality of life results. Additionally, this oncologic control should be interpreted with caution, as histologic risk classification changed over the study period and may not represent a modern cohort39. The present study took nearly 9 years to accrue, which we believe is due to the rare nature of SRP and unfamiliar nature of SRP to referring physicians. By comparison, the largest studies to date are based on chart reviews over 20–30-year period22-25.
Additionally, complications data were not available to inform which patients were more likely to experience a complication with respect to radiation exposure or surgeon volume. Despite limitations, the prospective and multiinstitutional nature of this cohort with long-term oncologic outcomes adds to its strength, which differs from most of the recent series that have been published that have been retrospective, represent single institution experiences, and have shorter follow-up.
Conclusions
SRP is a feasible treatment for radiation-resistant or recurrent CaP in some men. Modern surgical techniques continue to be associated with significant peri-operative complication rates. Nevertheless, SRP may benefit carefully selected patients through durable oncologic control.
Acknowledgements: The research for CALGB 9687 (Alliance) was supported, in part, by grants from the National Cancer Institute (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, MD, Chair) and to the Alliance Statistics and Data Center (Daniel J. Sargent, Ph.D., CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. The following institutions participated in this study: Cedars-Sinai Medical Center, Los Angeles, CA, Armando Giuliano, MD Duke University Medical Center, Durham, NC, Jeffrey Crawford, MD, supported by CA47577 Memorial Sloan-Kettering Cancer Center, New York, NY, Clifford A. Hudis, MD, supported by CA77651 University of California at San Francisco, San Francisco, CA, Charles J. Ryan, MD, supported by CA60138 University of Chicago, Chicago, IL, Hedy L. Kindler, MD, supported by CA41287 University of Massachusetts Medical School, Worcester, MA, William V. Walsh, MD, supported by CA37135 University of North Carolina at Chapel Hill, Chapel Hill, NC, Thomas C. Shea, MD, supported by CA47559.
Compliance with ethical standards: Conflict of interest – The authors declare that they have no conflict of interest.
Authors: James L. Mohler1, Susan Halabi2, Stephen T. Ryan3, Ali Al-Daghmin1, Mitchell H. Sokoloff4, Gary D. Steinberg5, Ben L. Sanford6, James A. Eastham7, Philip J. Walther8, Michael J. Morris7, Eric J. Small9
1. Roswell Park Cancer Institute, Buffalo, NY CA59518, United States
2. Department of Biostatistics and Bioinformatics and Alliance Statistics and Data Center, Duke University, Durham, NC CA33601, United States
3. University of California San Diego, La Jolla, CA, United States
4. University of Massachusetts Medical School, Worchester, MA, United States
5. University of Chicago, Chicago, IL CA41287, United States
6. Alliance Statistics and Data Center, Duke University, Durham, NC CA33601, United States
7. Memorial Sloan Kettering Cancer Center, New York, NY CA77651, United States
8. Duke University Medical Center, Durham, NC CA47577, United States
9. University of California at San Francisco, San Francisco, CA CA60138, United States
References
1. Meng MV, Elkin EP, Latini DM, Duchane J, Carroll PR. Treatment of patients with high risk localized prostate cancer: results from cancer of the prostate strategic urological research endeavor (CaPSURE). J Urol. 2005;173:1557–61.
2. Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22:2141–9.
3. Kupelian PA, Buchsbaum JC, Patel C, Elshaikh M, Reddy CA, Zippe C et al. Impact of biochemical failure on overall survival after radiation therapy for localized prostate cancer in the PSA era. Int J Radiat Oncol Biol Phys. 2002;52:704–11.
4. Zelefsky MJ, Fuks Z, Hunt M, Lee HJ, Lombardi D, Ling CC et al. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166:876–81.
5. Shipley WU, Thames HD, Sandler HM, Hanks GE, Zietman AL, Perez CA et al. Radiation therapy for clinically localized prostate cancer: a multi-institutional pooled analysis. JAMA. 1999;281:1598–604.
6. Zelefsky MJ, Chan H, Hunt M, Yamada Y, Shippy AM, Amols H. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006;176:1415–9.
7. Pollack A, Zagars GK, Antolak JA, Kuban DA, Rosen II. Prostate biopsy status and PSA nadir level as early surrogates for treatment failure: analysis of a prostate cancer randomized radiation dose escalation trial. Int J Radiat Oncol Biol Phys. 2002;54:677–85.
8. Crook J, Malone S, Perry G, Bahadur Y, Robertson S, Abdolell M. Postradiotherapy prostate biopsies: what do they really mean? Results for 498 patients. Int J Radiat Oncol Biol Phys. 2000;48:355–67.
9. Borghede G, Aldenborg F, Wurzinger E, Johansson KA, Hedelin H. Analysis of the local control in lymph-node staged localized prostate cancer treated by external beam radiotherapy, assessed by digital rectal examination, serum prostate-specific antigen and biopsy. Br J Urol. 1997;80:247–55.
10. Holzman M, Carlton CE Jr, Scardino PT. The frequency and morbidity of local tumor recurrence after definitive radiotherapy for stage C prostate cancer. J Urol. 1991;146:1578–82.
11. Fuks Z, Leibel SA, Wallner KE, Begg CB, Fair WR, Anderson LL et al. The effect of local control on metastatic dissemination in carcinoma of the prostate: long-term results in patients treated with 125I implantation. Int J Radiat Oncol Biol Phys. 1991;21:537–47.
12. Brenner PC, Russo P, Wood DP, Morse MJ, Donat SM, Fair WR. Salvage radical prostatectomy in the management of locally recurrent prostate cancer after 125I implantation. Br J Urol. 1995;75:44–7.
13. Pontes JE, Montie J, Klein E, Huben R. Salvage surgery for radiation failure in prostate cancer. Cancer. 1993;71:976–80.
14. Ahlering TE, Lieskovsky G, Skinner DG. Salvage surgery plus androgen deprivation for radioresistant prostatic adenocarcinoma. J Urol. 1992;147:900–2.
15. Stein A, Smith RB, deKernion JB. Salvage radical prostatectomy after failure of curative radiotherapy for adenocarcinoma of prostate. Urology. 1992;40:197–200.
16. Rainwater LM, Zincke H. Radical prostatectomy after radiation therapy for cancer of the prostate: feasibility and prognosis. J Urol. 1988;140:1455–9.
17. Nguyen PL, D’Amico AV, Lee AK, Suh WW. Patient selection, cancer control, and complications after salvage local therapy for postradiation prostate-specific antigen failure: a systematic review of the literature. Cancer. 2007;110:1417–28.
18. Mitchell JM. Urologists’ use of intensity-modulated radiation therapy for prostate cancer. N Engl J Med. 2013;369:1629–37.
19. Chade DC, Shariat SF, Cronin AM, Savage CJ, Karnes RJ, Blute ML et al. Salvage radical prostatectomy for radiation-recurrent prostate cancer: a multi-institutional collaboration. Eur Urol. 2011;60:205–10.
20. Stephenson AJ, Eastham JA. Role of salvage radical prostatectomy for recurrent prostate cancer after radiation therapy. J Clin Oncol. 2005;23:8198–203.
21. Grossfeld GD, Li YP, PL DP, Carroll PR. Patterns of failure after primary local therapy for prostate cancer and rationale for secondary therapy. Urology. 2002;60:57–62. discussion62-3
22. Touma NJ, Izawa JI, Chin JL. Current status of local salvage therapies following radiation failure for prostate cancer. J Urol. 2005;173:373–9.
23. Ward JF, Sebo TJ, Blute ML, Zincke H. Salvage surgery for radiorecurrent prostate cancer: contemporary outcomes. J Urol. 2005;173:1156–60.
24. Stephenson AJ, Scardino PT, Bianco FJ Jr, DiBlasio CJ, Fearn PA, Eastham JA. Morbidity and functional outcomes of salvage radical prostatectomy for locally recurrent prostate cancer after radiation therapy. J Urol. 2004;172:2239–43.
25. Bianco FJ Jr, Scardino PT, Stephenson AJ, Diblasio CJ, Fearn PA, Eastham JA. Long-term oncologic results of salvage radical prostatectomy for locally recurrent prostate cancer after radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:448–53.
26. Heidenreich A, Richter S, Thuer D, Pfister D. Prognostic parameters, complications, and oncologic and functional outcome of salvage radical prostatectomy for locally recurrent prostate cancer after 21st-century radiotherapy. Eur Urol. 2010;57:437–43.
27. Sanderson KM, Penson DF, Cai J, Groshen S, Stein JP, Lieskovsky G et al. Salvage radical prostatectomy: quality of life outcomes and long-term oncological control of radiorecurrent prostate cancer. J Urol. 2006;176:2025–31. discussion 2031-2
28. Krupski TL, Saigal CS, Hanley J, Schonlau M, Litwin MS. Patterns of care for men with prostate cancer after failure of primary treatment. Cancer. 2006;107:258–65.
29. Van Der Poel HG, Moonen L, Horenblas S. Sequential treatment for recurrent localized prostate cancer. J Surg Oncol. 2008;97:377–82.
30. Leonardo C, Simone G, Papalia R, Franco G, Guaglianone S, Gallucci M. Salvage radical prostatectomy for recurrent prostate cancer after radiation therapy. Int J Urol. 2009;16:584–6.
31. Kaffenberger SD, Keegan KA, Bansal NK, Morgan TM, Tang DH, Barocas DA et al. Salvage robotic assisted laparoscopic radical prostatectomy: A single institution, 5-year experience. J Urol 2013;189:507–513.
32. Yuh B, Ruel N, Muldrew S, Mejia R, Novara G, Kawachi M et al. Complications and outcomes of salvage robot-assisted radical prostatectomy: a single-institution experience. BJU Int 2014; 113: 769–76.
33. Chauhan S, Patel MB, Coelho R, Liss M, Rocco B, Sivaraman AK et al. Preliminary Analysis of the Feasibility and Safety of Salvage Robot-Assisted Radical Prostatectomy After Radiation Failure: Multi-Institutional Perioperative and Short-Term Functional Outcomes. J Endourol 2011;25:1013–19.
34. Strope SA, Coelho M, Wood DP, Hollenbeck BK. Robot-assisted salvage prostatectomy: evaluation of initial patient-reported outcomes. J Endourol. 2010;24:425–7. Management of recurrent prostate cancer after radiotherapy: long-term results from CALGB 9687. . . 315
35. Boris RS, Bhandari A, Krane LS, Eun D, Kaul S, Peabody JO. Salvage robotic-assisted radical prostatectomy: initial results and early report of outcomes. BJU Int. 2009;103:952–6.
37. Pisters LL, Leibovici D, Blute M, Zincke H, Sebo TJ, Slezak JM et al. Locally Recurrent Prostate Cancer After Initial Radiation Therapy: A Comparison of Salvage Radical Prostatectomy Versus Cryotherapy. J Urol 2009;182:517–27.
38. Song J, Li M, Zagaja GP, Taxy JB, Shalhav AL, Al-Ahmadie HA. Intraoperative frozen section assessment of pelvic lymph nodes during radical prostatectomy is of limited value. BJU Int. 2010;106:1463–7.
39. Epstein JI, Allsbrook WC, Amin MB, Egevad LL, ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228–42.
Further Related Content: A Commentary from the Associate Editor of PCAN
