Basel, Switzerland (UroToday.com) Dr. Mark Rubin briefly shared his thoughts on the future of metastatic prostate cancer in regards to biomarkers. He began his talk with some definitions of prognostic and predictive biomarkers.
A prognostic biomarker is one that indicates an increased (or decreased) likelihood of a future clinical event, disease recurrence or progression in an identified population. Prognostic biomarkers are measured at a defined baseline, which may include a background treatment.
A predictive biomarker is used to identify individuals who are more likely to respond to exposure to a particular medical product or environmental agent. The response could be a symptomatic benefit, improved survival or an adverse effect.
Next, Dr. Rubin discussed some of the important features of advanced prostate cancer. Approximately 5%, 10%, and 20% of advanced prostate cancer patients have microsatellite instability (MSI) or mismatch repair gene mutations, germline mutations (such as BRCA), and DRM somatic-germline mutations, respectively.
Dr. Rubin believes that what we now require are:
- To overcome heterogeneity by using a liquid biopsy to overcome the limits of biopsying multiple metastases and capturing heterogeneity and /or serial biopsies1
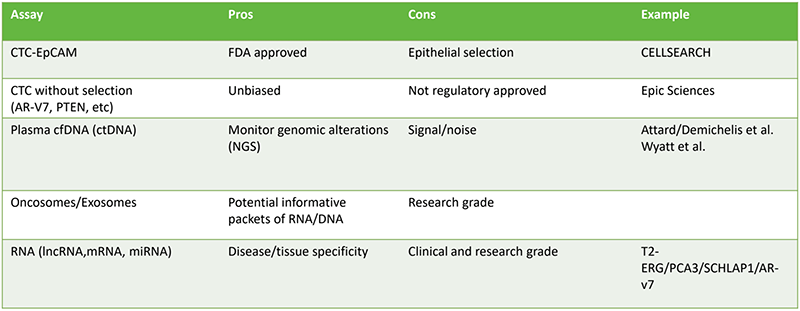
- We need to incorporate advanced biomarkers for advanced prostate cancer – these are non-invasive approaches to monitor the evolution of prostate cancer. Some of the currently used biomarkers are shown in table 1. It is important that these biomarkers are predictive, prognostic and reproducible.
Table 1 – Currently used biomarker tests:
Dr. Rubin concluded his talk giving a summary of some of the many studied biomarkers that are ready/appear promising. These include the following:
- Microsatellite instability (MSI testing).
- DNA repair status (assay for BRCA1/2, ATM, PALB2).
- Loss of androgen receptor or lack of response to the androgen receptor (ARV7 mutations).
- cfDNA amount has been shown to be associated with prognosis.
- PTEN loss – possible response to AKT inhibitor (de Bon CCR 2018).
- CDK12 loss – possible response to checkpoint blockade.
- Loss of TP53/RB1 – short duration of response to androgen receptor therapy – possibly predictive response to platinum.
- CTC heterogeneity – response to docetaxel vs. androgen receptor therapy.
- Pathology – phenotype for NEPC response to platinum.
- PSMA expression response to PSMA drug therapies.
- DLL3 expression response to chemo conjugate.
These should continue to be studied and incorporated in prospective randomized trials.Presented by: Mark A. Rubin, MD, Principal Investigator, Director DBMR, University of Bern, Switzerland
Written by: Hanan Goldberg, MD, Urology Department, SUNY Upstate Medical University, Syracuse, New-York, USA @GoldbergHanan at the 2019 Advanced Prostate Cancer Consensus Conference (APCCC) #APCCC19, Aug 29 – 31, 2019 in Basel, Switzerland
References:
- Schweizer MT, Antonarakis ES. Liquid biopsy: Clues on prostate cancer drug resistance. Science translational medicine. Nov 4 2015;7(312):312fs345.
