Barcelona, Spain (UroToday.com) Four large, randomized Phase 3 clinical trials (S-TRAC, ASSURE, PROTECT, and ATLAS) evaluated adjuvant VEGF tyrosine kinase inhibitors in patients with resected renal cell carcinoma (RCC). Only S-TRAC met the primary endpoint of improvement in disease-free survival (DFS). None of these studies demonstrated improvement in overall survival (OS). Dr. Tim Eisen presents the primary efficacy results from the SORCE trial, an international, randomized, double-blind Phase 3 trial that evaluated adjuvant sorafenib for RCC at intermediate or high risk of relapse.
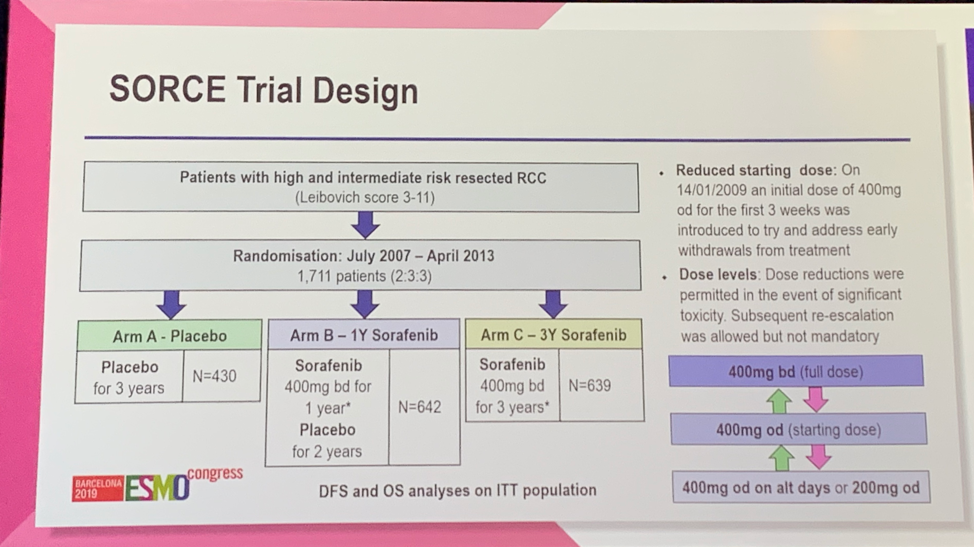
Eligible patients had intermediate or high risk resected RCC as determined by a Leibovich score of 3-11.1 1,711 patients were randomized 2:3:3 to placebo (Arm A), 1 year of sorafenib followed by 2 years of placebo (Arm B), or 3 years of sorafenib (Arm C). Of note, the initial statistical plan was to compare at least one year of treatment vs placebo (Arm A vs Arm B+C), however when the results of the ASSURE and S-TRAC trials were published, the primary analysis plan was revised to compare three years of sorafenib (Arm C) to placebo (Arm A) to focus on the question of longer exposure. In the initial design, the starting dose of sorafenib was 400 mg twice daily. Given high rates of early withdrawal from treatment due to treatment-emergent adverse events (TEAEs), the starting dose was reduced to 400 mg daily for the first 3 weeks with dose adjustments as required (shown below). The primary endpoints were DFS and OS in the intention-to-treat population.
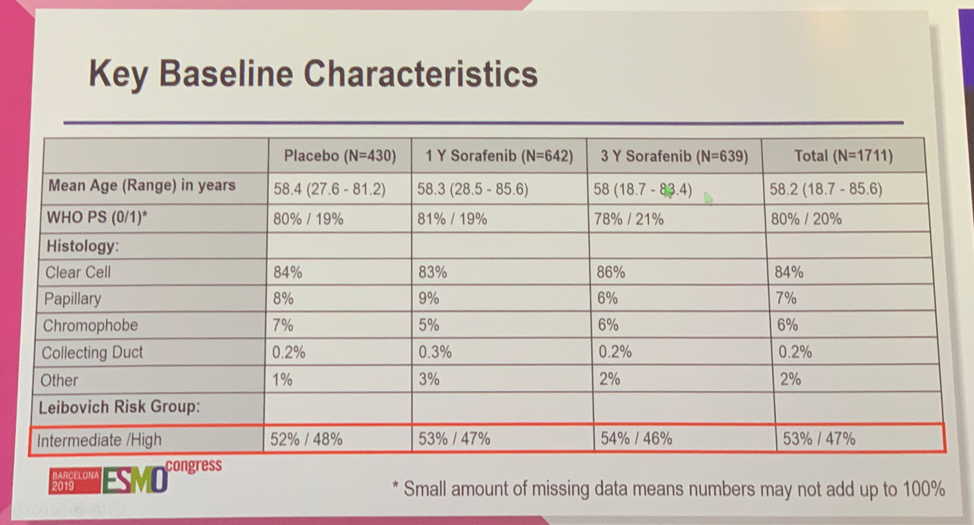
Baseline characteristics were well-balanced across the treatment groups. Approximately 80% of patients had a performance status of 0 and approximately 85% had clear cell histology. Slightly more than 50% of patients in all groups were intermediate risk and slightly less than 50% of patients were high risk.
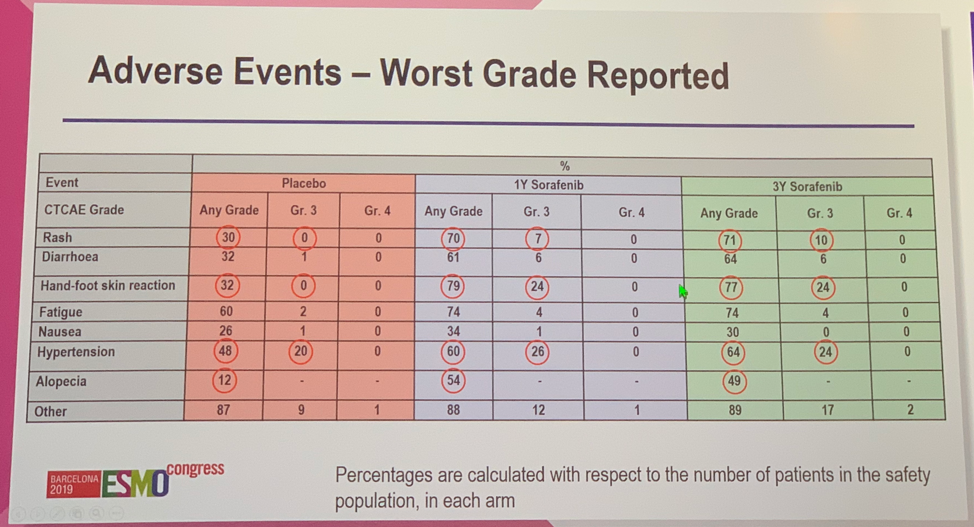
The primary efficacy analysis found that there were no significant differences in outcomes between patients receiving placebo, 1 year of sorafenib, or 3 years of sorafenib for 5-year DFS (67% vs 67% vs 65%), 10-year DFS (54% vs 55% vs 53%), and 10-year OS (69% vs 69% vs 70%). Notably, 55% of patients receiving placebo completed protocol treatment compared with only 33% and 24% of patients randomized to receive 1 year or 3 years of sorafenib. This discrepancy was thought to be driven primarily by excessive toxicity of the experimental treatment, which occurred in 30% and 34% of patients treated with 1 or 3 years of sorafenib and only 5% of patients who received placebo. Subgroup analysis of DFS and OS limited to patients with high-risk of relapse or only clear cell histology did not show any difference compared to the overall cohort.
The most common TEAE among patients receiving sorafenib was hand-foot skin reaction, which occurred in 77-79% of patients with 24% of patients experiencing grade 3 severity. Hypertension was another commonly observed TEAE, however, this was seen in patients receiving placebo as well, though rates were not as high as those on the experimental arm.
Dr. Eisen concluded that the SORCE trial failed to meet its primary endpoint as up to three years of sorafenib did not improve DFS or OS in patients with resected RCC at intermediate or high risk of relapse compared to placebo. Active surveillance remains the global standard of care for patients at intermediate or high risk of recurrence following nephrectomy.
Presented by: Tim Q. Eisen, PhD, MB, BChir, Professor of Medical Oncology at the University of Cambridge; Head of Oncology Early Clinical Projects, AstraZeneca
Written by: Jacob Berchuck, MD, Medical Oncology Fellow at the Dana-Farber Cancer Institute (Twitter: @jberchuck) at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct 2019 in Barcelona, Spain
References:
- Leibovich BC, Cheville JC, Lohse CM, et al. A scoring algorithm to predict survival for patients with metastatic clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. J Urol. 2005 Nov;174(5):1759-63.
