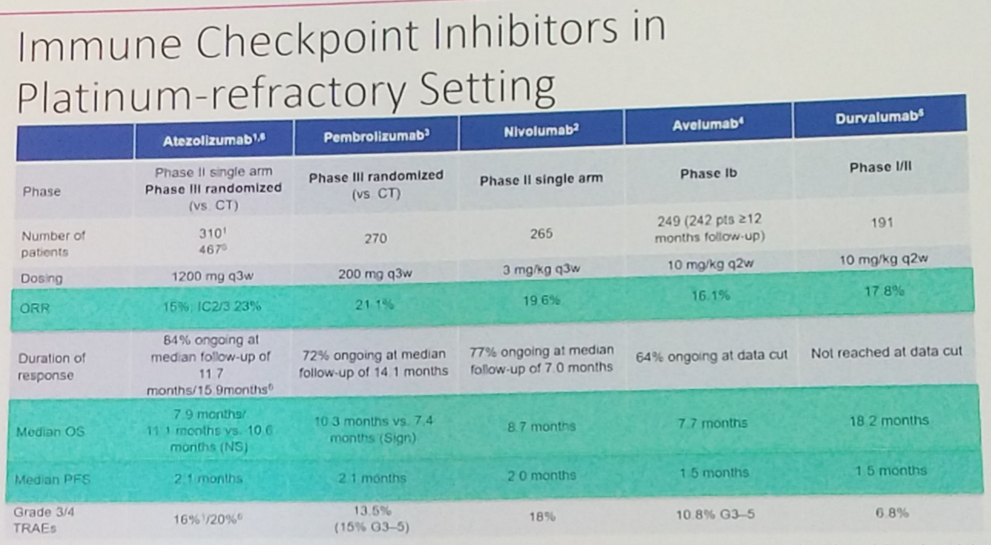
Barcelona, Spain (UroToday.com) Dr. Joaquim Bellmunt provided an overview of the current state of immune checkpoint inhibition in advanced urothelial cancer, emphasizing the clinical uses based on available data as well as future directions for clinical development of immunotherapy and novel immune-oncology combinations. He began by summarizing the results from the pivotal trial of the 5 anti-PD-1 and anti-PD-L1 immune checkpoint inhibitors which have been FDA approved in the platinum-refractory (2L) setting. While response rates were modest – ranging from 15% with atezolizumab in IMvigor210 cohort 2 to 21.1% with pembrolizumab in KEYNOTE-045 – responses were remarkably durable, with the majority ongoing at last follow-up. It was on the basis of the duration of these responses – far longer than had been observed historically with second-line chemotherapy – that all 5 of these agents were approved.
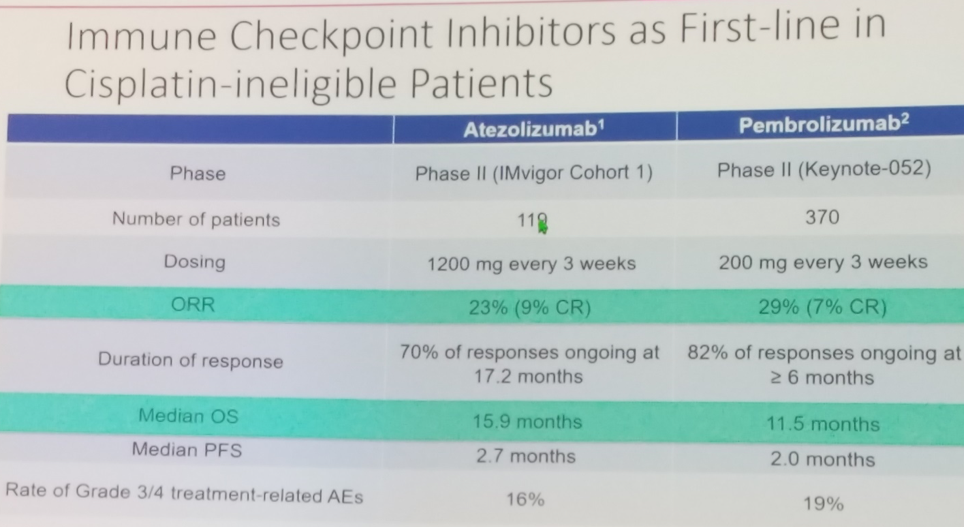
In the first-line cisplatin-ineligible setting, both the PD-L1 antibody atezolizumab and the PD-1 antibody pembrolizumab are approved as monotherapy in patients whose tumors express high levels of the biomarker PD-L1. However, this paradigm may be expected to change shortly given the report of improved progression-free survival with combination chemo-immunotherapy in the first-line setting in IMvigor130.
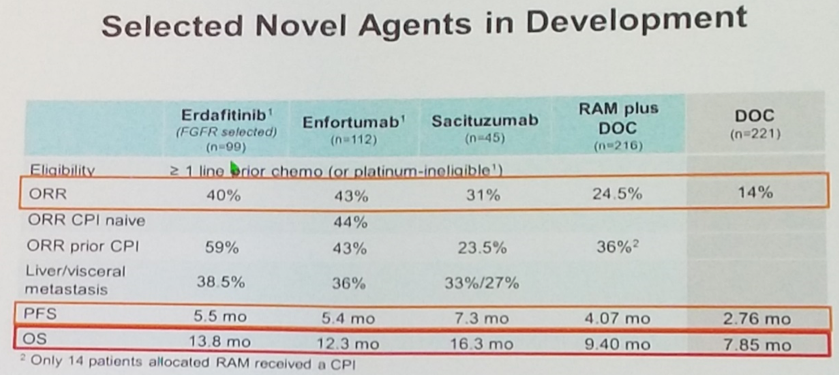
Dr. Bellmunt next moved on to discuss combinations, emphasizing the recent data of various targeted therapies as single agents which may be combined with checkpoint blockade in ongoing and future studies. Specifically, he pointed to two antibody-drug conjugates – the nectin-4-based ADC enfortumab vedotin and the trop-2-based ADC sacituzumab govitecan – which have both demonstrated relatively high response rates of 43% and 31% respectively as monotherapy in pretreated populations. In addition, he pointed to erdafitinib, a small molecule pan-FDGR inhibitor which was just recently FDA approved for the treatment of metastatic urothelial cancer with specific FGFR2 or FGFR3 alterations.
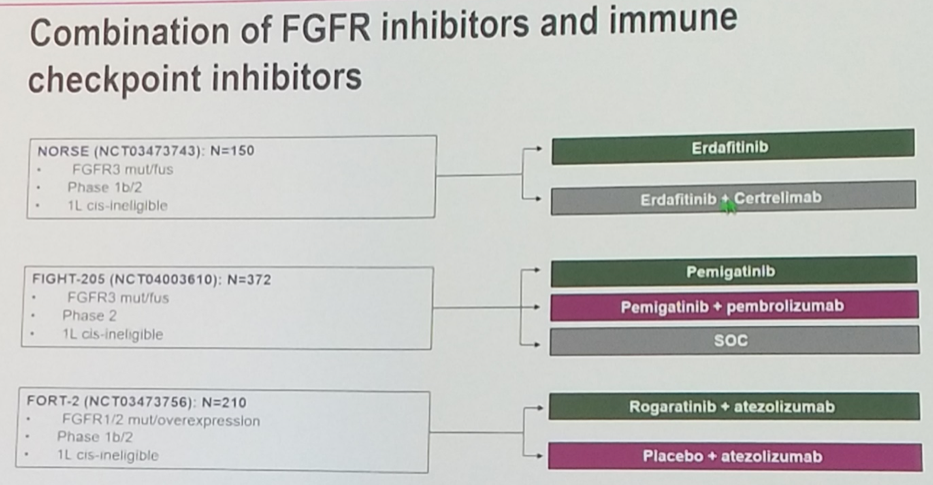
With respect to IO combinations for which data are available in urothelial carcinoma, a number of trials are ongoing to assess the efficacy of chemotherapy plus immunotherapy – most notable IMvigor130 which demonstrated improved progression-free survival with combination therapy. In addition, combination immunotherapy with CTLA-4 antibodies in addition to anti-PD-1 or anti-PD-L1 also show promising results. The PD-L1 antibody durvalumab combined with the CTLA-4 antibody tremelimumab achieved a 29.4% response rate, while the anti-CTLA-4 + anti-PD-1 combination of ipilimumab with nivolumab demonstrated a remarkable 38% response rate with an ipilimumab 3 mg/kg plus nivolumab 1 mg/kg dosing regimen – both in the platinum-refractory setting.
Several studies are also investigating the combination of immunotherapy with various FGFR blocking agents.
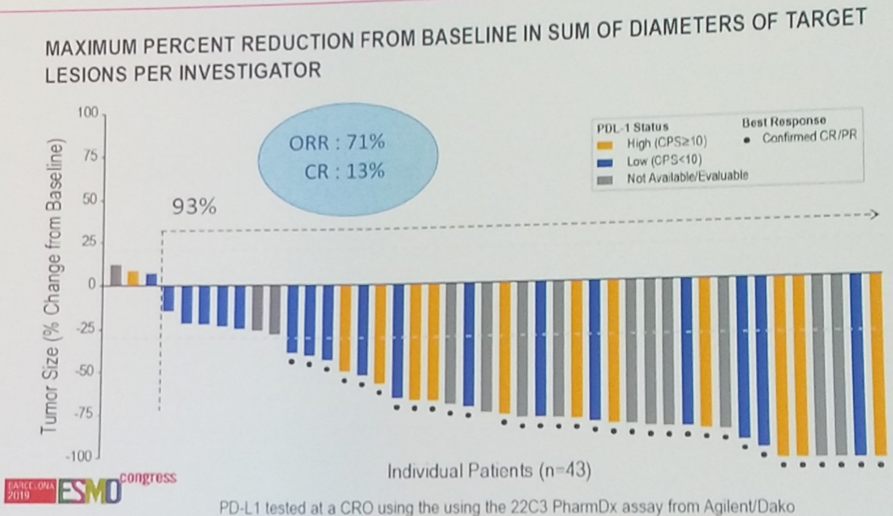
Combination PD-1 blockade with the antibody drug conjugate enfortumab vedotin is being studied in EV-103, which recently reported a very impressive 71% objective response rate with this novel IO combination.
In summary, checkpoint blockade immunotherapy has transformed the treatment landscape in metastatic urothelial cancer. Combination chemo-immunotherapy may soon become standard first-line therapy, and ongoing trials are seeking to assess the safety and efficacy of combining PD-1/PD-L1 blockade with FGFR targeted therapy, antibody drug conjugates, and CTLA-4 immune checkpoint inhibition.
Presented by: Joaquim Bellmunt, MD, PhD, Associate Professor of Medicine, Harvard Medical School, Genitourinary Oncologist, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA
Written by: Michael Lattanzi, MD, Medical Oncology Fellow, Memorial Sloan Kettering Cancer Center, Twitter: @MikeLattanzi at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct 2019 in Barcelona, Spain
