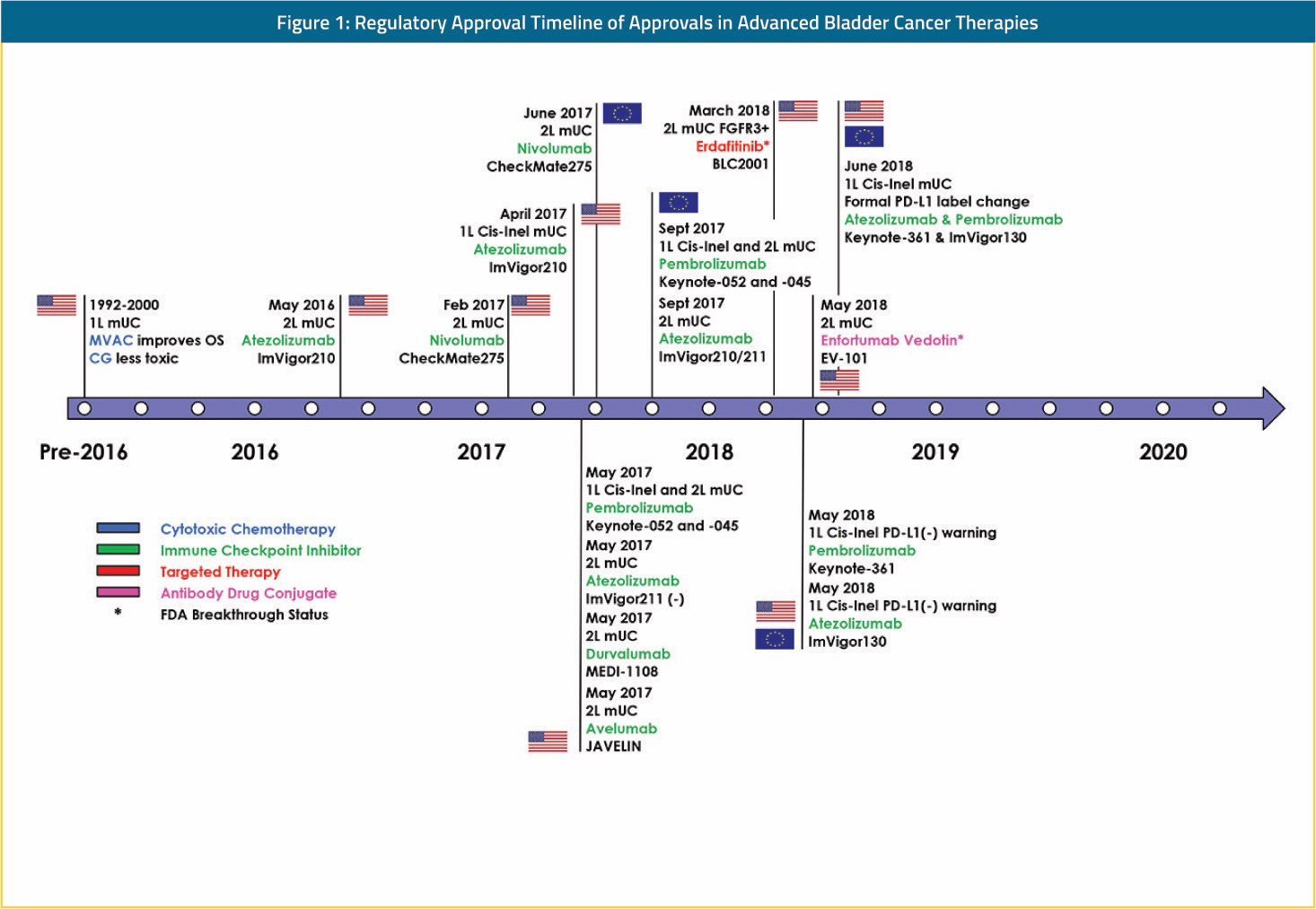
Recent years have seen an explosive rate of transformative advances in both pre-clinical and clinical urothelial carcinoma research. With the public dissemination of comprehensive molecular data from The Cancer Genome Atlas (TCGA) urothelial carcinoma cohort, the global urothelial carcinoma research community now has the initial road map of the key biological themes that drive carcinogenesis, growth, invasion, and metastasis.1 In the clinic, durable tumor responses in a minority of post-platinum metastatic urothelial carcinoma patients treated with PD-1/PD-L1 targeting immune checkpoint inhibitors (CPIs) has resulted in the FDA approval of 5 new CPI agents (atezolizumab, nivolumab, pembrolizumab, durvalumab, avelumab) since 2016.2-6 Furthermore, promising clinical activity has been demonstrated with fibroblast growth factor receptor (FGFR) inhibitors (erdafitinib) in metastatic patients with tumors harboring activating tumor FGFR alterations and with anti-body drug conjugates (enfortumab vedotin) regardless of tumor genomic alterations.7,8 These new drug approvals and breakthrough designations depicted in Figure 1 have provided much-needed additional therapy options and improved outcomes for metastatic urothelial carcinoma patients.
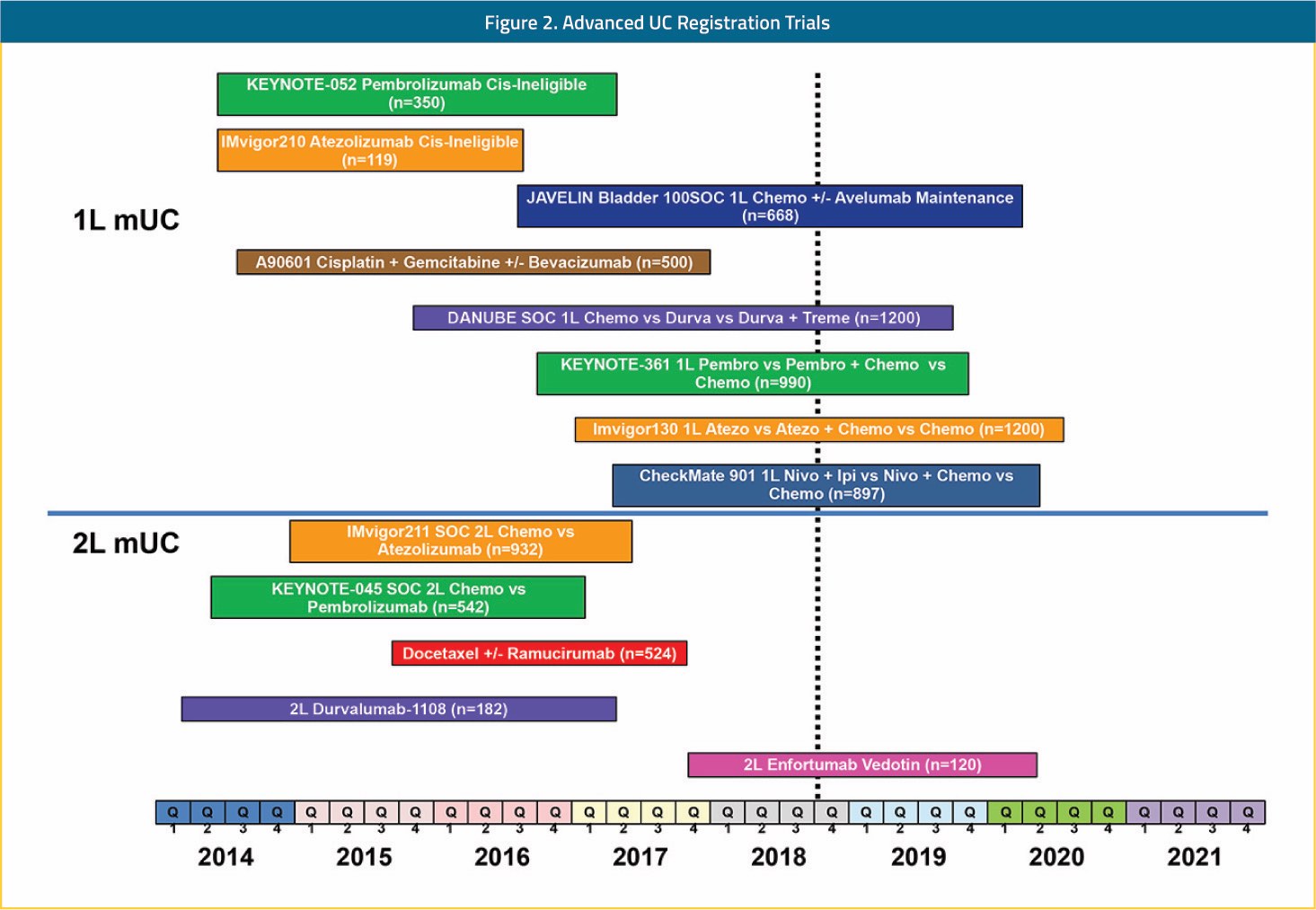
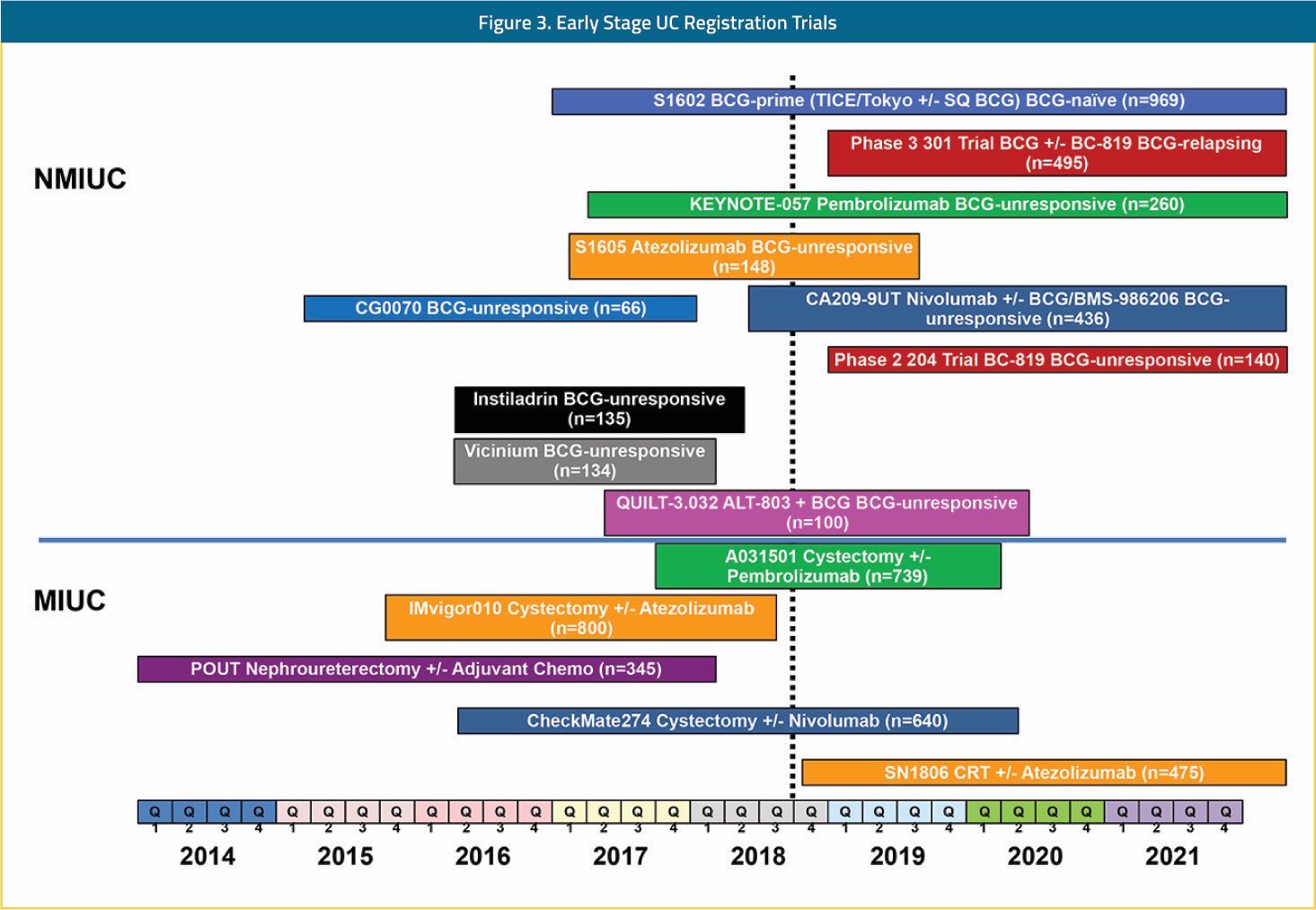
The impact of these rapid advances for both urothelial carcinoma patients and the research community is difficult to overstate. Prior to this recent renaissance, investment in urothelial carcinoma drug development was scant from both the private and public sectors. The belief that urothelial carcinoma patients were too frail to be treated safely, that urothelial carcinoma trials could not accrue well, and that urothelial carcinoma did not possess therapeutically exploitable biology or targets was a widely held dogma. Besides achieving their primary goal of improving the lives of patients with urothelial carcinoma, the FDA approvals shown in Figure 1 resoundingly demonstrated to the world that urothelial carcinoma is a fertile ground for new drug registration strategies. This point has forever changed urothelial carcinoma from a patient population with few clinical trial options to a population with an abundance of trials to choose from across all stages of the disease. Shown in Figure 2 is a snapshot of recently completed, ongoing, and planned FDA registration trials in urothelial carcinoma. With multiple registration trials anticipated to read out in the next few years in the front-line metastatic, adjuvant, and BCG-unresponsive non-muscle invasive populations, continued rapid evolution in standards of care and scientific discovery are anticipated. While change can evoke fear of the unknown, in the case of urothelial carcinoma this should not be the case. Indeed, our biggest unknown about the changes that will follow is how far further we will improve the outlook for patients with urothelial carcinoma. For this fact, urothelial carcinoma patients, families, and physician providers are justifiably hopeful and tremendously thankful.