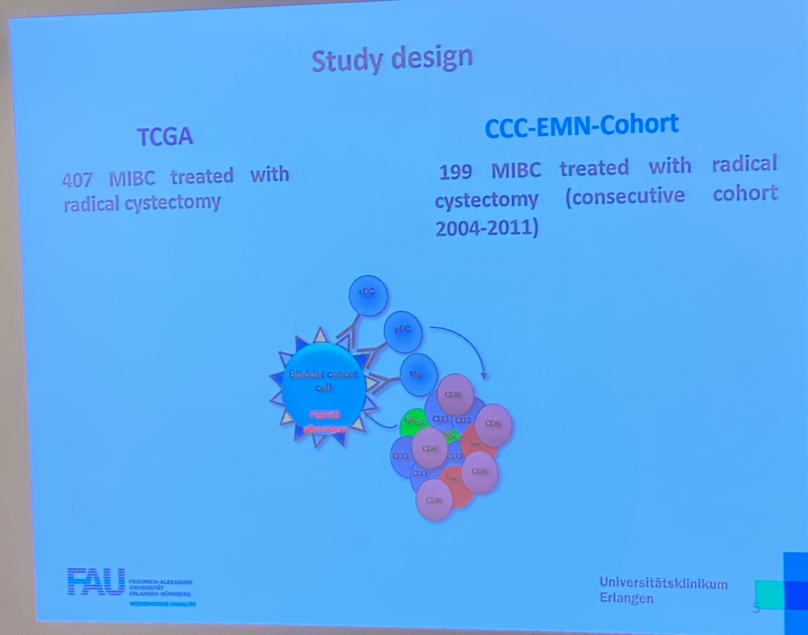
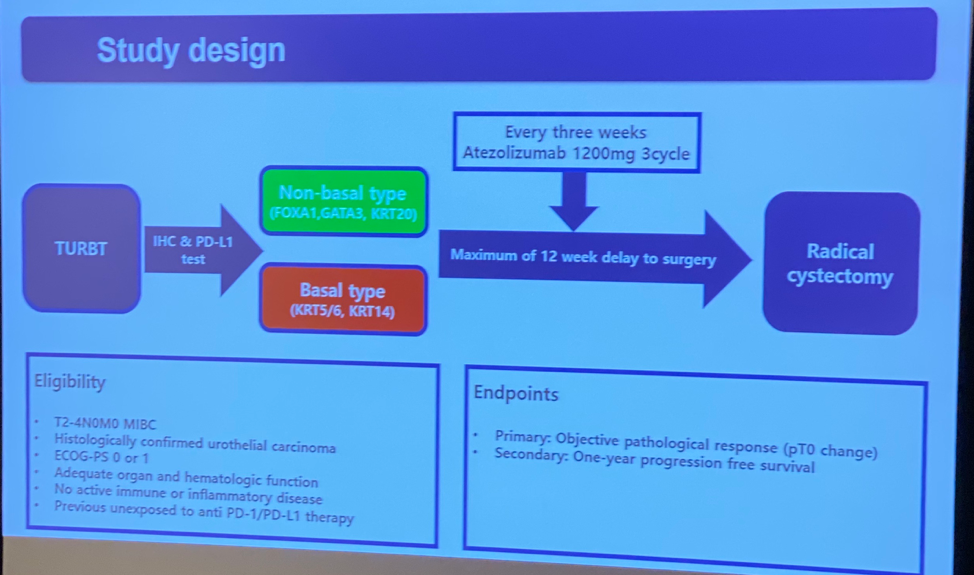
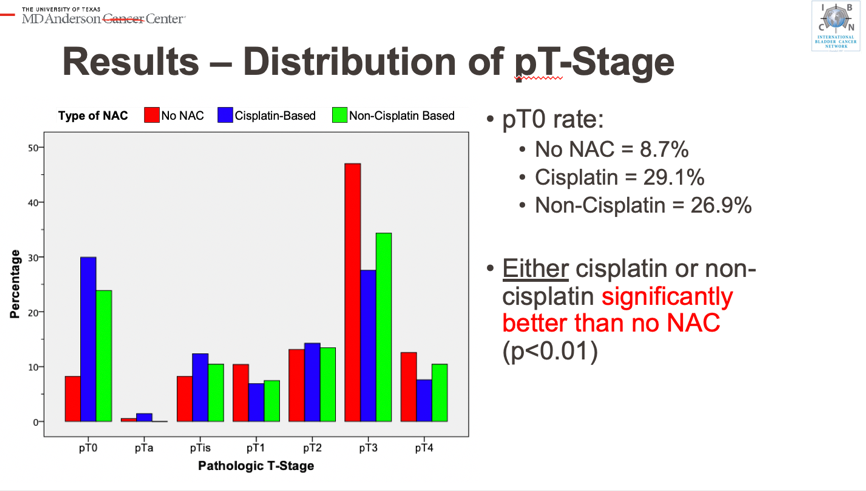
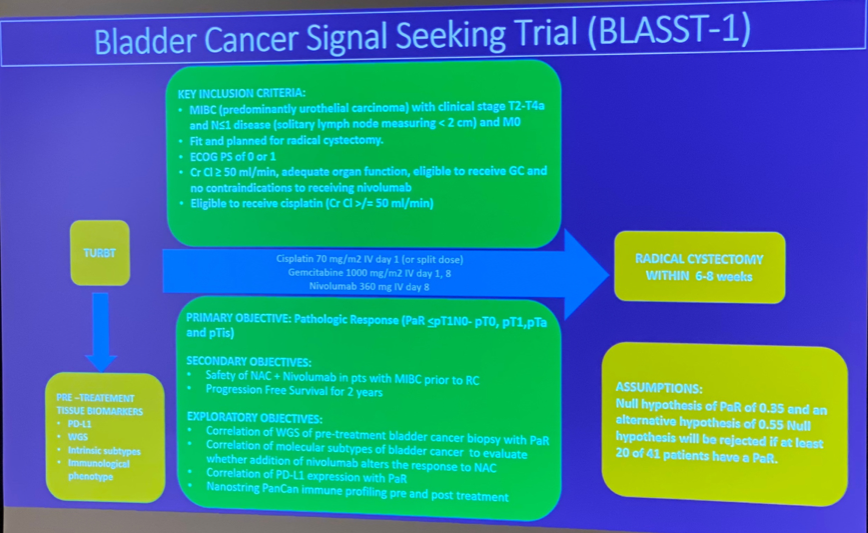
Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma: How to Apply New Evidence in Clinical Practice.
Cytoreductive nephrectomy (CN) followed by systemic therapy had been considered the standard of care for metastatic renal cell carcinoma (mRCC) patients since two clinical trials established its role during the cytokines era. With introduction of new and effective drugs, such as vascular endothelial growth factor-targeted therapies, the role of CN started to be challenged. Retrospective […]