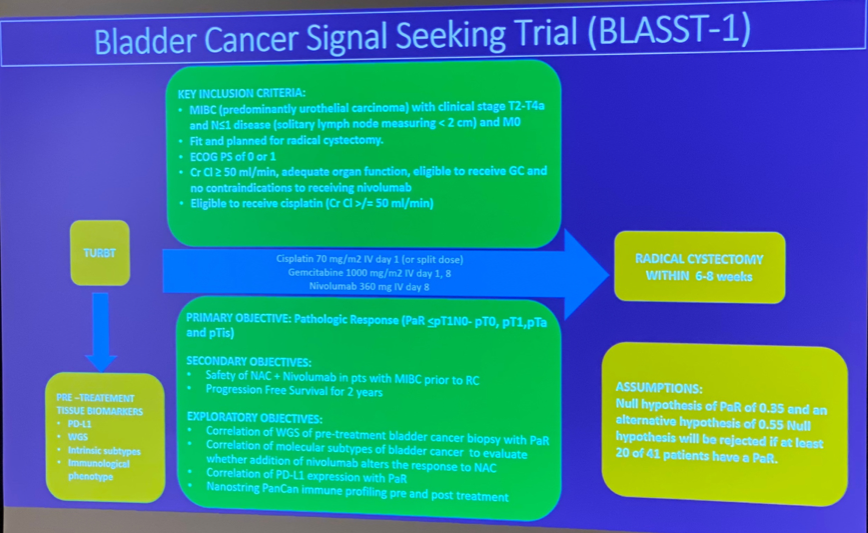
Aarhus, Denmark (UroToday.com) In this oral abstract session, Dr. Shilpa Gupta from the Cleveland Clinic Taussig Cancer Institute presented data on behalf of the Bladder Cancer Signal Seeking Trial (BLASST-1) group. The BLASST-1 trial is a phase II trial investigating the efficacy and safety of nivolumab with gemcitabine-cisplatin (GC) as a neoadjuvant option for patients with muscle-invasive bladder cancer (NCT03294304).
Eligible patients included those with cT2-T4a, N<=1, M0 MIBC who were cystectomy-eligible. Patients received GC and nivolumab for 4 cycles followed by radical cystectomy within 8 weeks. The primary endpoint was a pathologic response (defined as <=pT1, N0). A total of 41 patients were enrolled; interim analyses were performed when 29 patients underwent RC. The pathologic response was observed in 24 out of 29 patients (82.7%), with no reported deaths from treatment. Adverse events included grade 3-4 neutropenia, thrombocytopenia, and renal insufficiency. There were no surgical complications reported related to therapy, and no delay in time to radical cystectomy (RC).
Abstract take-home message:
- Neoadjuvant nivolumab with gemcitabine-cisplatin for cT2-T4a, N<=1, M0 MIBC was associated with an 83% pathologic response rate (defined as p<=T1) (preliminary/interim)
Presented by: Shilpa Gupta, MD, Department of Hematology and Medical Oncology, Cleveland Clinic Taussig Cancer Institute, Cleveland, Ohio
