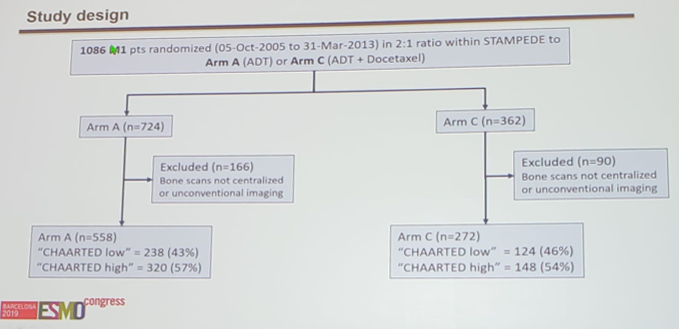
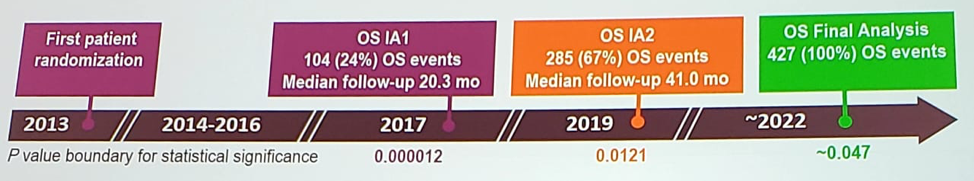
ESMO 2019: Docetaxel for Hormone-naïve Prostate Cancer: Results from Long-term Follow-up of Metastatic Patients in the STAMPEDE Randomized Trial and Sub-group Analysis by Metastatic Burden
Barcelona, Spain (UroToday.com) The STAMPEDE trial consortium previously reported that upfront docetaxel improved overall survival (OS) for patients starting long-term androgen deprivation therapy (ADT).1 Over a median follow-up of 43 months (IQR 30–60), there were 415 deaths in the control group, with a median OS of 71 months (IQR 32-not reached (NR)) for standard of care, […]