Barcelona, Spain (UroToday.com) In the phase III placebo-controlled SPARTAN study, apalutamide with ongoing androgen deprivation therapy (ADT) significantly improved metastasis-free survival (MFS) (HR 0.28, 95% CI, 0.23-0.35), time to symptomatic progression (HR 0.45, 95% CI 0.32-0.63), and progression-free survival on second therapy compared with placebo with ongoing ADT in patients with non-metastatic castration-resistant prostate cancer (nmCRPC).1
Importantly, 76 patients (~19%) who were receiving placebo crossed over to receive apalutamide. At the primary analysis for MFS, apalutamide was associated with improved overall survival (OS) (HR 0.70, 95% CI, 0.47-1.04; p = 0.07) and time to initiation of cytotoxic chemotherapy. However, the OS results were immature, with only 104 of 427 events (24%) required for the prespecified final OS analysis. During the prostate cancer session at the 2019 European Society for Medical Oncology annual meeting (ESMO), Dr. Matthew Smith, on behalf of the SPARTAN investigators, presented updated data from this phase III trial, specifically the second interim analysis for OS after 285 events (65% of required events).
For SPARTAN, 1,207 patients with nmCRPC and prostate-specific antigen doubling time of ≤ 10 months were randomized 2:1 to receive apalutamide (240 mg QD) or placebo, with ongoing ADT. The OS effect of apalutamide vs placebo was assessed using a group sequential testing procedure with O’Brien-Fleming-type alpha spending function. Of note, the required p value for statistical significance at the second interim analysis was 0.0121. OS was analyzed by Kaplan-Meier method and Cox model.
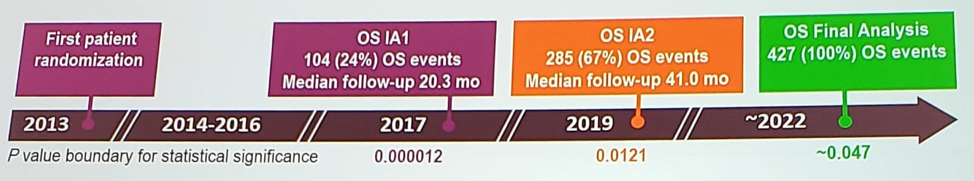
At 41 months of median follow-up and 285 OS events, apalutamide was associated with improved OS compared with placebo (HR 0.75, 95% CI, 0.59-0.96; p = 0.0197), the p-value just higher than the prespecified p-value for the significance of 0.0121.
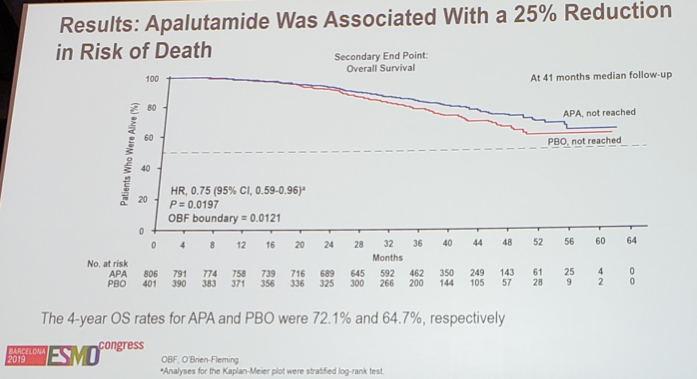
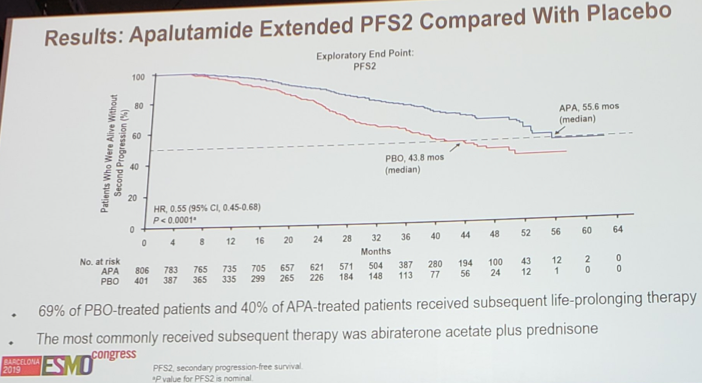
Results of the apalutamide treatment effect for OS was also consistent across subgroups. The 4-yr OS rates for apalutamide was 72.1% and 64.7% for the placebo group. At the second interim analysis, the proportion of patients who received subsequent life-prolonging therapy was 69% in the placebo group and 40% in the apalutamide group. Time to PFS2 also favored apalutamide: HR 0.55, 95% CI 0.45-0.68 (median 55.6 months vs 43.8 months).
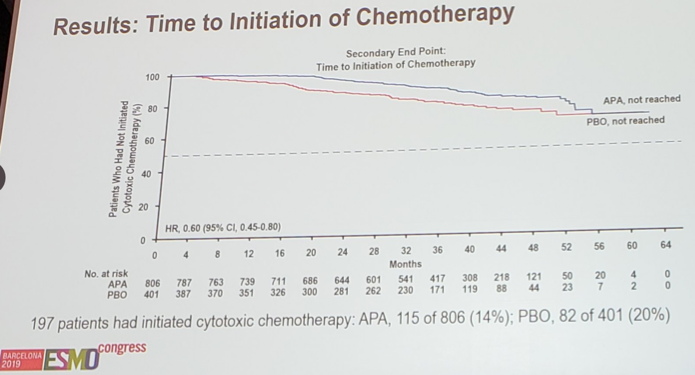
At this analysis, 197 patients had initiated chemotherapy: 115 of 806 (14%) for apalutamide, compared to 82 of 401 (20%) for placebo. Although the median time to initiation of chemotherapy was not yet reached for either group, the KM curve shows benefit for apalutamide (HR 0.60, 95% CI 0.45-0.80):
Rates of discontinuation due to progressive disease and adverse events were 34% and 14%, for the apalutamide group, and 74% and 8% for the placebo group. Importantly, the rates of treatment-emergent adverse events for apalutamide at the second interim analysis were similar to the rates previously reported at the initial interim analysis.
Dr. Smith conclude his presentation of the second interim analysis of SPARTAN with the following take-home messages:
- Apalutamide was associated with a 25% reduction in risk of death compared with placebo
- This OS benefit for apalutamide was observed despite crossover of placebo patients to apalutamide and higher rates of subsequent life-prolonging therapy for placebo patients
- The apalutamide safety profile remained unchanged with longer follow-up
- These results further support apalutamide as a standard of care option for patients with high-risk nmCRPC
Clinical trial identification: NCT01946204.
Presented by: Matthew R. Smith, MD, Ph.D., Director of the Genitourinary Oncology Program at Massachusetts General Hospital Cancer Center and an Associate Professor of Medicine at Harvard Medical School, Boston, Massachusetts, USA
Co-Authors: F. Saad2, S. Chowdhury3, S. Oudard4, B. Hadaschik5, J. Graff6, D. Olmos7, P. Mainwaring8, J.Y. Lee9, H. Uemura10, P. De Porre11, A. Smith12, K. Zhang12, A. Lopez-Gitlitz13, E. Small14
2. Hospital St. Luc du CHUM, Montreal, CA
3. Guy’s and St. Thomas’ Hospital NHS Trust, London, UK
4. Hospital European George Pompidou, Paris, FR
5. University Hospital of Essen, Essen, DE
6. OHSU Knight Cancer Institute, Portland, US
7. Spanish National Cancer Research, Málaga, ES
8. ICON Cancer Care, Brisbane, AU
9. St. Mary’s Hospital of Catholic University, Seoul, KR
10. Yokohama City University Medical Center, Yokohama, JP
11. Janssen EMEA Belgium, Beerse, BE
12. Janssen Research & Development, San Diego, US
13. Janssen Research & Development, Los Angeles, US
14. UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, US
Reference:
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct, 2019 in Barcelona, Spain
