Basel, Switzerland (UroToday.com) Dr. Morgans presented the IRONMAN project, which is the international registry for men with advanced prostate cancer.
There are an estimated 1.3 million new cases of prostate cancer and 359,000 prostate cancer deaths globally each year. This is a rapidly evolving clinical landscape and there is variability in guidelines and clinical practice in advanced disease. There is an urgent need to identify optimal treatment patterns and strategies for patients. This registry also includes molecular subtyping and patient-reported outcomes measures (PROMs).
The objectives of this impressive registry include:
- To describe the practice patterns of therapeutic agents for the treatment of advanced prostate cancer internationally
- To assess whether specific treatment patterns are associated with clinically significant adverse events
- To evaluate the potential interactions with concomitant medications or demographic factors
- To identify associations between treatment sequences or combinations and overall survival
- To define the patient’s experience of advanced prostate cancer and identify unmet needs in their treatment
- Identify clinical and molecular disease subtypes that predict response to individual treatments, combinations or sequences
The patient population of the IRONMAN study includes males 21 years and older with histological/cytological confirmed advanced prostate cancer with serum PSA > 20 ng/ml. The disease state cohorts include either metastatic hormone-sensitive prostate cancer (mHSPC) patients, or castrate-resistant prostate cancer (CRPC) patients.
According to the study design, at baseline, the following will be collected: medical history, prior cancer treatment, concomitant medications, lab results, and physician questionnaire. At follow-up, further collection of data will occur which will include changes in cancer treatment, concomitant medications, clinically significant adverse events, relevant lab results and physician questionnaire results.
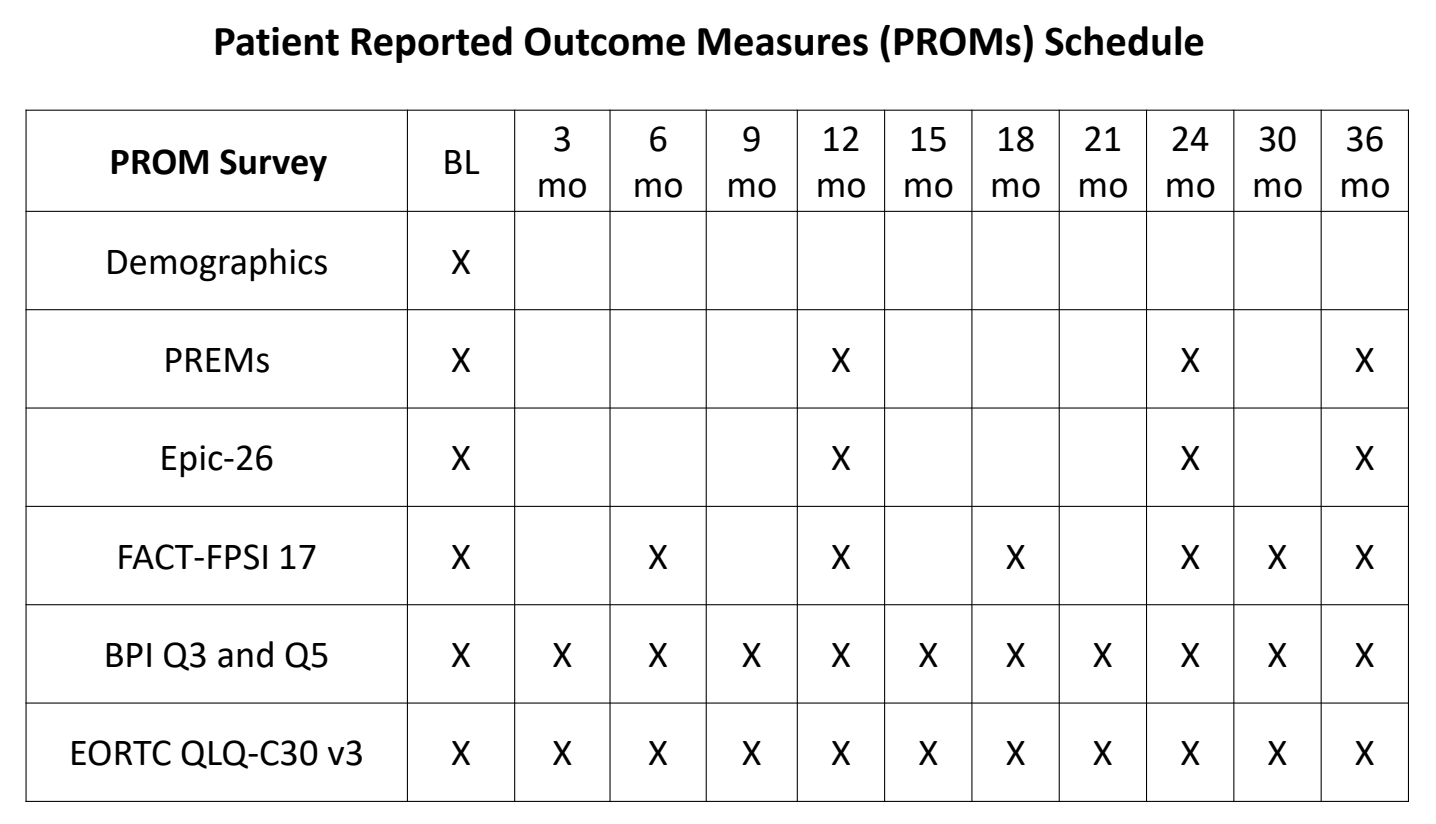
PROMs will also be collected at baseline and during follow-up (Table 1). This will include demographic data, quality of life assessments at baseline, every 3 months for the first 2 years and every 6 months thereafter. There is also an ongoing sub-study aiming to evaluate whether PROMs are used to trigger a follow-up contact from a clinician when a patient reports high levels of ill health and if this results in an improved quality of life and overall survival.
Table 1. Patient-reported outcome measures
The physician questionnaire will be completed by the patient’s healthcare provider at baseline, during the first change in treatment, at each subsequent change in treatment, and finally at treatment discontinuation. The questionnaires will focus on what made the physician decide to stop treatment and move on to the next treatment.
As mentioned before, biospecimens will be collected as well during this study. This will include the collection of whole blood, plasma, cell-free DNA, buffy coat, and RNA. Biospecimen collection will occur at enrollment, at first change of treatment due to disease progression, at 12 months follow-up, and at each subsequent change in treatment due to progression.
The study includes several working groups including a diversity working group, which will highlight the strategic selection of additional US sites focusing on regions with racial/ethnic diversity and high prostate cancer mortality rates. Additionally, other regions/countries will be explored including Africa and the Caribbean, Norway, Japan, and France.
We eagerly await the advancement of this study and the interesting results and data that it is predicted to produce.
Presented by: Alicia Morgans, MD, Northwestern University, Chicago, Illinois
Written by: Hanan Goldberg, MD, Urology Department, SUNY Upstate Medical University, Syracuse, New-York, USA, Twitter: @GoldbergHanan at the 2019 Advanced Prostate Cancer Consensus Conference (APCCC) #APCCC19, Aug 29 – 31, 2019 in Basel, Switzerland
