Basel, Switzerland (UroToday.com) Dr. Himisha Beltran gave an overview looking into the future of oligometastatic disease, and where she predicts we will be in two years’ time.
The current definition of oligometastatic disease is based on the number of metastases (1-5). Importantly, novel imaging modalities such as PET-PSMA have resulted in defining a worse disease state. For example, if conventional imaging (CT and bone scans) are negative but the PET-PSMA recognizes 3 lesions, patients are upstaged to metastatic setting. Furthermore, when 3 lesions e identified in standard imaging, but if many more are seen in PET PSMA imaging, the patient is upstaged from low-volume to high volume metastatic disease.
The goals of care should be determined by biology and stratified by:
- Low metastatic potential.
- Intermediate or heterogeneous disease.
- Systemic disease.
For low metastatic potential – the goals should be delaying ADT, coming off systemic therapy, and perhaps even cure. Not all metastatic lesions come from the same clone of the primary tumor.1 It is not clear if molecular alterations in the primary tumor predict patterns of spread and the relative indolence of certain metastatic lesions. The primary tumor can continue to seed metastases, which provides us the reason why we should probably treat the primary tumor even in metastatic disease. The STAMPEDE2 and HORRAD3 trials point to greater benefit of removing the primary tumor in patients with low-volume disease. This potentially might be due to the greater contribution of the primary tumor clones.4
For intermediate or heterogeneous disease – An example of this is when one lesion progresses, and the others are stable (Figure 1). This could be explained by the fact that there is different biology across metastases. Could this mean that the different metastases came from different primary tumors? Or maybe the reason is the acquisition of resistance mutations during progression (Figure 2)? The way to combat heterogeneity in the same patient is with multimodal therapy. The goal should be to stay on the same systemic therapy and slow down progression.
For systemic disease – local/focal therapy may have no impact and the systemic therapy may need to be intensified. There are many methods to capture the underlying systemic disease. These include using molecular imaging, ctDNA, circulating tumor cells, exosomes, and other means. It is still not clear what exactly we are looking for – tumor burden, micrometastases, alteration in oncogenes/tumor suppressor genes, or metastases markers.
One method to capture tumor burden noninvasively is by using novel imaging modalities such as the PSMA PET, which is highly sensitive. However, it is not clear if we can use these advanced imaging tools to predict metastatic patterns before “detecting” them. For instance, in bone metastases – what we might see, might not be what is truly underneath (which is more aggressive biology). In contrast, for pulmonary metastases, what we see may not be what is underneath (which is a more indolent disease).
Figure 1 – Stable and progressing metastatic lesions:
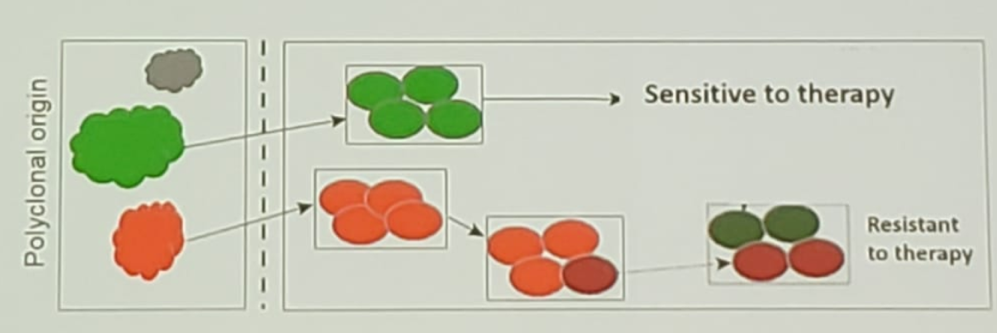
Figure 2 -Acquisition of resistance mutations during progression:
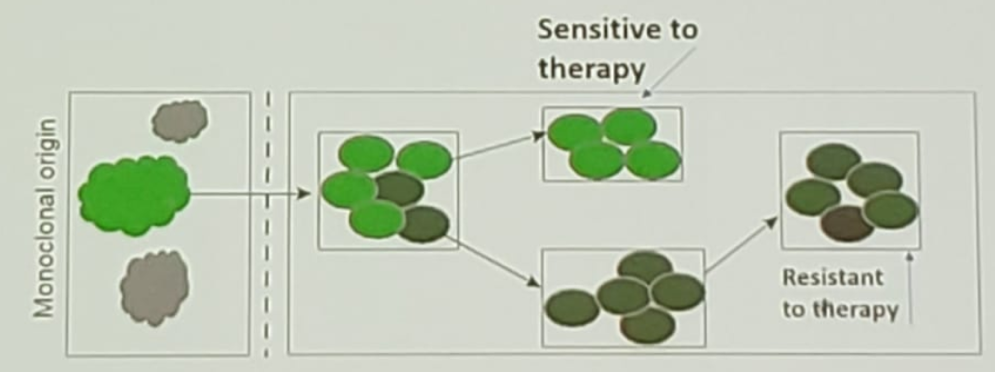
The concept of metastases to metastases seeding leads to further intra-patient heterogeneity. It is difficult to capture by single-site biopsy. The therapeutic implications of this concept are that multimodal therapy needs to be used. Today it is possible to use liquid biopsies to detect intra-patient clonal heterogeneity, with whole-exome sequencing of matched plasma ctDNA and metastatic tumor burden. Using circulating tumor cells, we can capture the evolving heterogeneity, with serial testing identifying evolution/emergence of clones/subclones and evolution patterns.
According to Dr. Beltran, in two years, when the Advanced Prostate Cancer Consensus Conference (APCCC) 2021 occurs, we will have more data regarding how we have altered the natural history of oligometastatic prostate cancer. Hopefully, we will be able to answer some or perhaps all the following questions:
- What happens to patients who progress after local therapy for oligometastatic prostate cancer?
- If more oligometastases develop, do we just give more local therapy? Is there a need for systemic therapy at some threshold? And what is that threshold?
- When a widespread disease has developed, is the timing of this important? Was this delayed by local therapy? Is this important and does it have any significance?
Dr. Beltran summarized her talk and predicted that in two years from now we will have refined clinical definitions for disease stratification. We will have also nominated molecular biomarkers to capture biologic disease states, and we will have defined goals of care and improved patient selection.
Presented by: Himisha Beltran, MD, Medical Oncologist, Dana Farber Cancer Institute, Harvard Medical School, Boston, Massachusetts, United States
Written by: Hanan Goldberg, MD, Urology Department, SUNY Upstate Medical University, Syracuse, New-York, USA @GoldbergHanan at the 2019 Advanced Prostate Cancer Consensus Conference (APCCC) #APCCC19, Aug 29 – 31, 2019 in Basel, Switzerland
References:
- Kneppers J, Krijgsman O, Melis M, et al. Frequent clonal relations between metastases and non-index prostate cancer lesions. JCI Insight. 2019;4(2):e124756.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet (London, England). Dec 1 2018;392(10162):2353-2366.
- Boeve LMS, Hulshof M, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. European urology. Mar 2019;75(3):410-418.
- Gundem G, Van Loo P, Kremeyer B, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. Apr 16 2015;520(7547):353-357.
