Basel, Switzerland (UroToday.com) Dr. Kim Chi from Vancouver presented an update on the utilization of PARP inhibitors during the management of castration-resistant prostate cancer (CRPC) session at the 2019 Advanced Prostate Cancer Consensus Conference (APCCC).
Dr. Chi started by highlighting that August 7, 2019, AstraZeneca announced that Lynparza® (olaparib) met the primary endpoint of significantly increasing the time patients selected for BRCA1/2 or ATM mutations live without radiographic disease progression versus standard of care treatment. As such, olaparib is the only PARP inhibitor with positive phase III results in four different cancer types: ovarian, breast, pancreatic, and now prostate.
It is now well-known that metastatic prostate cancer frequently harbors alterations in DNA damage repair pathway genes. Furthermore, PARP inhibition is synthetically lethal in homologous recombination repair-deficient cells. PARP1 plays a key role in ssDNA repair via binding to sites of DNA damage and serving as a platform to recruit DNA repair proteins. These proteins then add poly (ADP-ribose) units to the target proteins (PARylation). Inhibition of PARP1 catalytic activity prevents PARylation leading to replication fork collapse and dsDNA breaks, which would normally be repaired by homologous recombination.
The TOPARP-A Trial demonstrated that patients with metastatic castration-resistant prostate cancer (mCRPC) unresponsive to standard therapy who had DNA-repair defects responded to the PARP inhibitor olaparib1. Specifically, 16 of 49 patients (33%) had a response to olaparib, with 12 patients remaining on treatment for more than 6 months. Germline mutations have also been extensively studied in the prostate cancer arena. Among more than 700 patients with mCRPC who have had their germline DNA sequenced, more than 10% of patients have germline aberrations of DNA repair genes.
TOPARP-B was a phase II trial for patients with mCRPC preselected for putatively pathogenic DNA damage repair alterations2. Because TOPARP-A used a 400 mg olaparib dose and patients with breast cancer typically use a 300 mg dose, patients in TOPARP-B were randomized 1:1 to 400mg or 300mg of olaparib BID, aiming to exclude ≤ 30% response rate (RR) in either arm. The primary endpoint RR was defined as radiological response (RECIST 1.1) and/or PSA50% fall and/or CTC count conversion (Cellsearch; ≥ 5 to < 5), confirmed after 4-weeks. There were 98 patients (median age 67.6 years) randomized, with 92 patients treated and evaluable for the primary endpoint. The overall RR was 54% (95%CI 39-69%), meeting threshold for primary endpoint) in the 400 mg cohort and 39% (95%CI 24-54%) in the 300 mg cohort. Over a median follow-up of 17.6 months, the overall median PFS (mPFS) was 5.4 months. Subgroup analyses per altered gene identified indicated response rates for BRCA1/2 of 83% (mPFS 8.1 months), PALB2 57% (mPFS 5.3 months), ATM 37% (mPFS 6.1 months), CDK12 25% (mPFS 2.9 months), and others [ATRX, CHEK1, CHEK2, FANCA, FANCF, FANCG, FANCI, FANCM, RAD50, WRN] 20% (mPFS 2.8 months). The highest PSA50% response rates were observed in the BRCA1/2 (77%) and PALB2 (67%) subgroups.
TRITON2 was a phase II study evaluating rucaparib 600 mg BID in patients with mCRPC associated homologous recombination repair gene alterations, initially presented at ESMO 20183. Among 85 patients in the interim analysis, 45 had a BRCA1/2 alteration, 18 had an ATM alteration, 13 had a CDK12 alteration, and 9 have other mutations in the HRR pathway. There were 46 of the 85 patients with measurable disease at more than 16 weeks follow up. For the patients with BRCA1/2 alteration, there was a 44% confirmed overall response rate and 51% confirmed PSA response rate. Patients harboring an ATM alteration did not receive any benefit: 0/18 confirmed PSA responses, and 0/5 confirmed RECIST responses. Results for CDK12 also showed that there were 0 out of 8 objective responses per RECIST and only 1 of 13 confirmed PSA responses.
The GALAHAD trial was a phase II study of niraparib in patients with mCRPC and biallelic DNA-repair gene defects, initially presented at ASCO 20194. For this study, a patient’s plasma sample was evaluated for detecting DNA repair defects, including mutations in BRCA1/2, ATM, FANCA, PALB2, CHEK2, BRIP1 or HDAC2. The primary endpoint was objective response rate (ORR) as defined by RECIST 1.1. A total of 120 patients with mCRPC were enrolled and of the 50 patients that had DNA repair defects, 29 had BRCA1/2 and 21 had non-BRCA mutations. The composite RR was 18/29 (62%) in the BRCA1/2 patients and non-BRCA 5/21 (23.8%). The objective RR was 6/16 (37.5%) in the BRCA1/2 patients and 2/15 (13.3%) in the non-BRCA group. Taken together, all of these trials have demonstrated encouraging results for patients with BRCA mutations, but have been quite futile for other tumor mutations, such as ATM and CDK12.
There are several other ongoing PARP inhibitor monotherapy mCRPC trials ongoing:
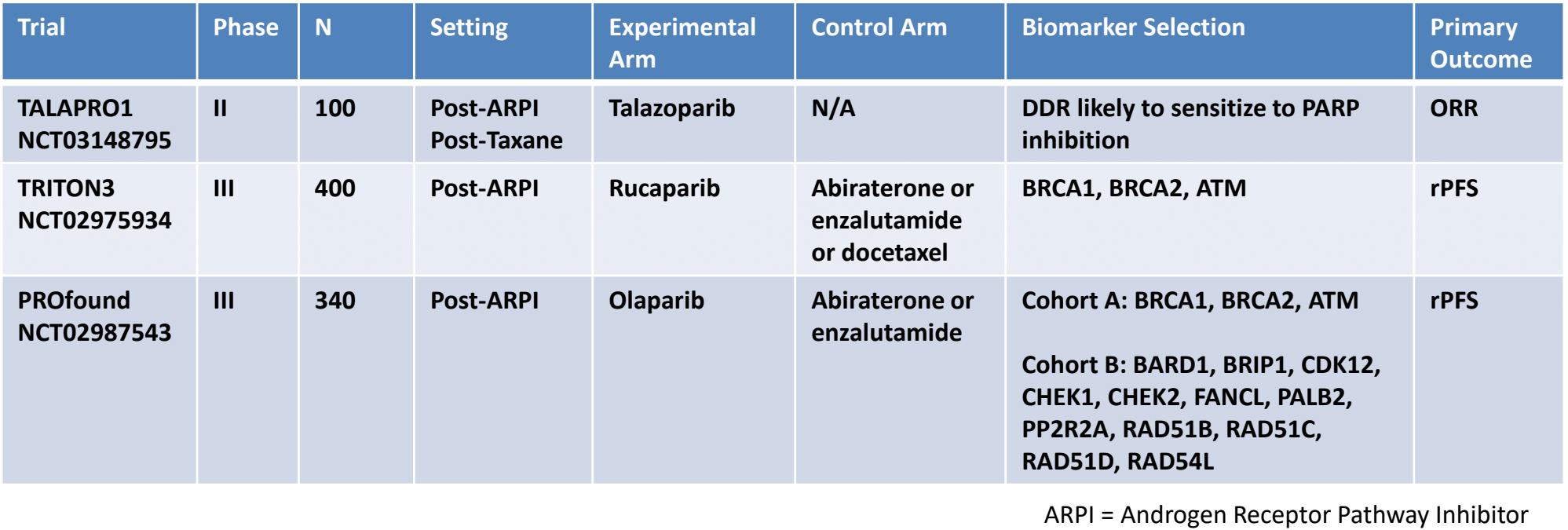
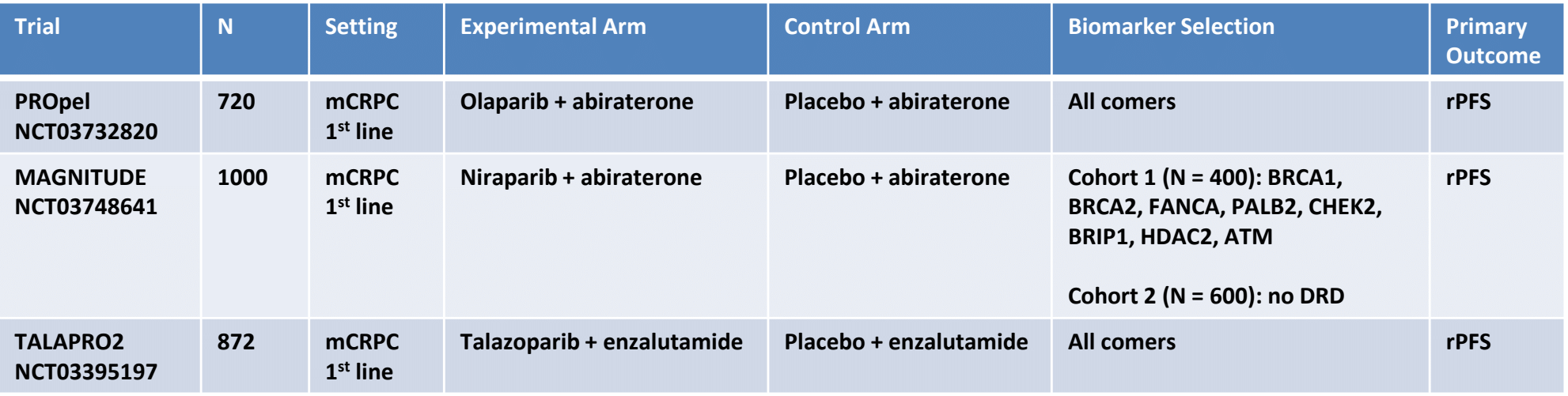
Combination PARP + AR pathway inhibition has also garnered significant interest. The androgen receptor promotes DNA damage repair, whereas ADT upregulates PARP-mediated repair pathways with synthetic lethality between ADT and PARP inhibition. PARP1 also regulates AR-mediated transcriptional activation. In 2018, results of phase 2 trial assessing olaparib with abiraterone were published in Lancet Oncology5. There were 142 patients randomly assigned to receive olaparib and abiraterone (n=71) or placebo and abiraterone (n=71). The median rPFS was 13.8 months (95% CI 10.8-20.4) with olaparib and abiraterone and 8.2 months (5.5-9.7) with placebo and abiraterone (HR 0.65, 95% CI 0.44-0.97, p = 0.034). One treatment-related death (pneumonitis) occurred in the olaparib and abiraterone group. Based on the results of this phase 2 study, the ongoing PROpel phase 3 trial will evaluate olaparib + abiraterone in the first line mCRPC setting.
Additional phase III combination PARP + AR pathway inhibition trials are ongoing:
There are also studies assessing combination PARP inhibition with immunotherapy. Preliminary results suggest that there is activity for durvalumab plus olaparib in mCRPC among men with and without DNA damage repair mutations. Additionally, KEYNOTE-365 Cohort A testing pembrolizumab + olaparib (n=28) found an ORR of 7%, with two patients exhibiting a partial response, and 13 patients with stable disease6. KEYLYNK-010 is also underway, which is assessing pembrolizumab + olaparib versus abiraterone acetate or enzalutamide in mCRPC patients.
Dr. Chi concluded this high-level talk with several summary points:
- Somatic and germline DNA damage repair gene defects are common in metastatic prostate cancer – we need to identify these patients
- PARP inhibitors as monotherapy have high levels of anti-tumor activity in mCRPC patients with identified alterations in DNA repair genes, especially BRCA2
- Phase 3 trials of combinations of PARP inhibitors with AR pathway inhibitors and immune check-point inhibitors are underway in selected and unselected patients
Presented by: Kim Chi, MD, Vancouver Prostate Centre, BC Cancer Agency, Vancouver, BC, Canada
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2019 Advanced Prostate Cancer Consensus Conference (APCCC) #APCCC19, Aug 29 – 31, 2019 in Basel, Switzerland
5. Clarke N, Wiechno P, Alekseev B, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: A randomized, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2018 Jul;19(7):975-986.
6. Yu, Evan Y., Christophe Massard, Margitta Retz, Ali Tafreshi, Joan Carles Galceran, Peter Hammerer, Peter CC Fong et al. “Keynote-365 cohort a: Pembrolizumab (pembro) plus olaparib in docetaxel-pretreated patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC).” (2019): 145-145.
