Barcelona, Spain (UroToday.com) Patients with metastatic urothelial carcinoma have limited options after disease progression on platinum-based chemotherapy and/or checkpoint inhibitors. Sacituzumab govitecan is an antibody-drug conjugate comprising a humanized anti-Trop-2 monoclonal antibody coupled to SN-38 via a unique hydrolysable linker. In the prior phase I/II IMMU-132-01 study, Sacituzumab govitecan showed significant clinical activity and manageable toxicity in 45 heavily pretreated metastatic urothelial patients. The overall response rate (ORR) was 31% and was 27% in patients with prior platinum-based chemotherapy/checkpoint inhibitors. At the 2019 European Society for Medical Oncology annual meeting (ESMO) proffered paper session, Dr. Scott Tagawa and colleagues presented initial results of TROPHY-U-01, a phase II trial assessing antitumor activity of Sacituzumab govitecan.
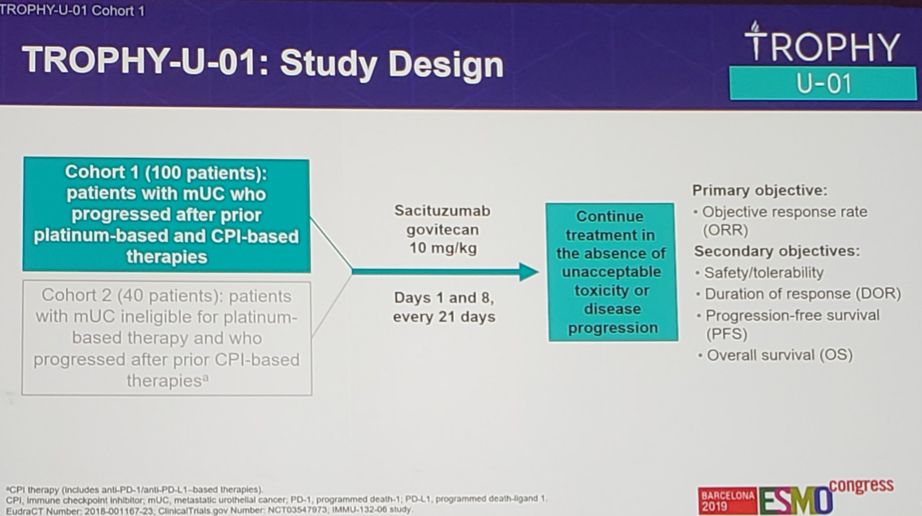
The TROPHY-U-01 study is a global, open-label, phase II trial evaluating the antitumor activity of Sacituzumab govitecan (10 mg/kg, days 1, 8 of 21-day cycles) in patients with metastatic urothelial carcinoma with measurable disease, ECOG performance status 0 or 1, and creatinine clearance ≥30 mL/min. This presentation is a pre-planned interim analysis based on investigator assessment per RECIST v1.1 from Cohort 1 – patients who progressed after both platinum-based chemotherapy and checkpoint inhibitors. Cohort 1 (n = 35) had a Simon two-stage design with a prespecified futility stopping rule of 11% objective response rate (ORR).
For this analysis, there were 35 patients with ≥1 post -baseline response assessment, the majority of which were male (80%), with a median age of 64 years (range 43-90 years), 77% of which had ≥1 Bellmunt risk factors, and having previously received a median of 3 (range 2-7) prior therapies. At a median follow-up of 4.1 months, the ORR was 29% with two confirmed complete responses, five confirmed partial responses, and three unconfirmed partial responses (all three ongoing and awaiting radiographic confirmation).
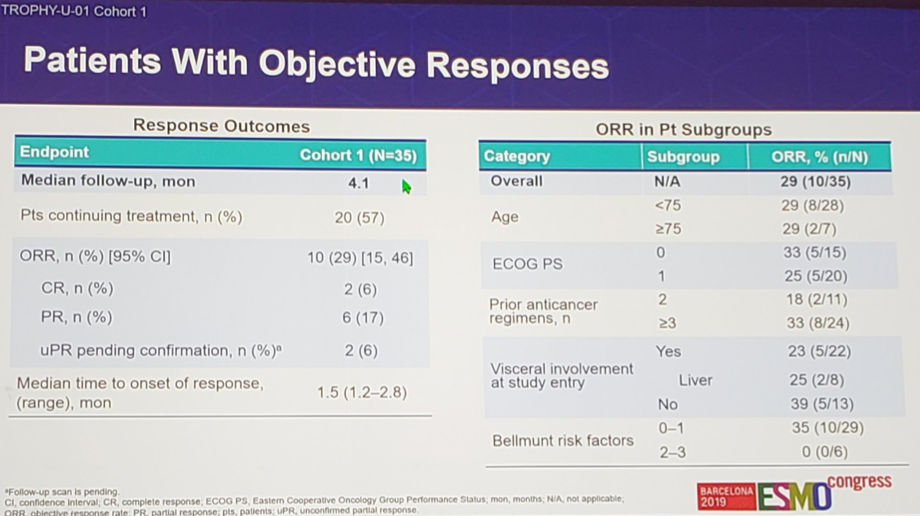
Importantly, 74% of patients had target lesion reduction. ORR was 25% in patients with liver involvement, and the safety profile was consistent with prior reports. Key grade ≥3 treatment-related adverse events were neutropenia (23%), anemia (17%), febrile neutropenia (11%), and diarrhea (11%), and there were no treatment-related deaths.
Dr. Tagawa concluded TROPHY-U-01 with several take-home messages:
- Interim Cohort 1 results surpassed the pre-specified futility stopping rule and enrollment continues, including patients in Cohort 2
- These findings confirm prior phase I/II study results of Sacituzumab govitecan as well-tolerated with significant antitumor activity in metastatic urothelial carcinoma patients after both prior platinum-based chemotherapy and checkpoint inhibitors
- In this population that continues to have a high unmet medical need despite recent progress, the ORR of 29% compares favorably with single-agent chemotherapy (ORR of 9%–14%)
Clinical Trial Information: NCT03547973
Presented by: Scott T. Tagawa, MD, The Richard A. Stratton Associate Professor in Hematology and Oncology, Weill Cornell Medical College, New York Presbyterian Hospital/Weill Cornell Medical Center, New York, New York
Co-Authors: S. Tagawa1, A. Balar2, D. Petrylak3, P. Grivas4, N. Agarwal5, C. Sternberg6, Q. Hong7, A. Gladden7, C. Kanwal7, P. Siemon-Hryczyk7, T. Goswami7, L. Itri7, Y. Loriot8
1. Weill Cornell Medical Center, New York, US
2. NYU Langone, New York, US
3. Yale Cancer Center, New Haven, US
4. University of Washington Seattle Cancer Care Alliance, Seattle, US
5. Huntsman Cancer Institute, Salt Lake City, US
6. Weill Cornell Medicine, New York, US
7. Immunomedics, Morris Plains, US
8. Institut Gustave Roussy, Villejuif, FR
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct, 2019 in Barcelona, Spain
