Barcelona, Spain (UroToday.com) The optimal timing of radiotherapy after radical prostatectomy for prostate cancer is uncertain. Supporters of adjuvant radiotherapy suggest that earlier treatment may be more effective, whereas those that support salvage radiotherapy suggest that this approach avoids unnecessary treatment. There are conflicting results from previous adjuvant radiotherapy trials: among 425 men with pT3N0M0 prostate cancer in SWOG S8794, those randomized to adjuvant radiotherapy had improved metastasis-free survival (MFS; HR 0.71, 95% CI 0.54-0.94) and OS (HR 0.72, 95% CI 0.55-0.96).1 However, in EORTC 22911, there was no difference in overall survival (OS) between adjuvant radiotherapy vs a wait-and-see policy (HR 1.18, 95% CI 0.91-1.53).2 At the 2019 European Society for Medical Oncology annual meeting (ESMO), prostate cancer session, Dr. Chris Parker presented the initial results of the RADICALS-RT trial, comparing the efficacy and safety of adjuvant radiotherapy versus an observation policy with salvage radiotherapy for PSA failure.
For RADICALS-RT, patients with post-op PSA ≤0.2 ng/ml and ≥1 risk factor (pT3/4, Gleason 7-10, positive margins or pre-op PSA ≥10 ng/ml) were randomized ≤ 22 weeks after radical prostatectomy to adjuvant or observation + salvage radiotherapy for PSA failure (PSA ≥0.1 ng/ml or three consecutive rises). Stratification factors were Gleason score, margin status, radiotherapy schedule (52.5Gy over 20 fractions, 66Gy over 33 fractions) and center.
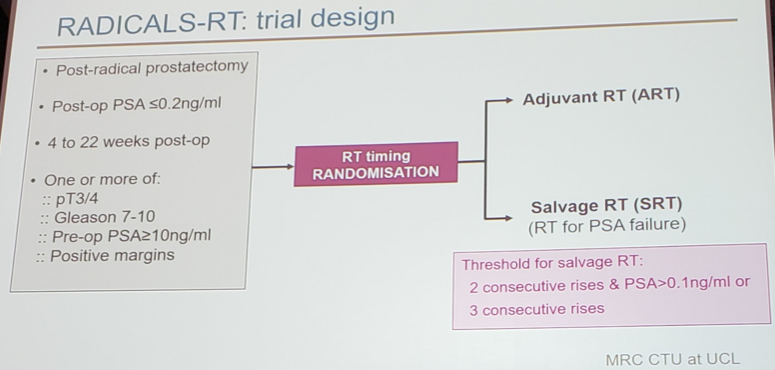
The primary outcome measure was freedom-from-distant metastases with >1200 patients needed for 80% power to detect an improvement from 90% to 95% at 10 years with adjuvant radiotherapy. Dr. Parker notes that it is too early to present results on the primary outcome measure, but data presented today notes the trial’s secondary outcome measures: biochemical PFS (any of PSA ≥0.4 ng/ml post-radiotherapy, PSA ≥2.0 ng/ml at any time, local/distant progression, deferred hormone therapy, or prostate cancer death), freedom-from-non-protocol hormone therapy, and safety (RTOG scale). The main analysis is planned after 67 freedom-from-distant metastases events. This early analysis of biochemical free survival and safety was undertaken to facilitate the ARTISTIC meta-analysis combining data from RADICALS, RAVES, and GETUG-17.
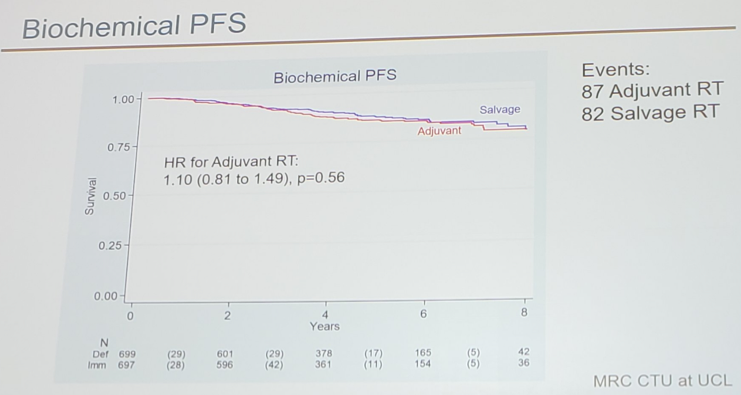
There were 1,396 patients randomized (697 to adjuvant radiotherapy and 699 to observation + salvage radiotherapy) from October 2007 to December 2016 (82% UK, 13% Denmark, 4% Canada, 1% Ireland). The median follow-up was 5 years, and among men in the adjuvant radiotherapy arm, 93% started radiotherapy within 5 months of radical prostatectomy. 33% of men in the observation + salvage radiotherapy arm started radiotherapy by 8 years after randomization. There were 26% of men in the adjuvant radiotherapy arm and 31% in the observation + salvage radiotherapy arm that reported hormone therapy with their radiotherapy. After 169 events, biochemical PFS at 5 years was 85% in the adjuvant radiotherapy arm vs 88% in the observation + salvage radiotherapy arm: HR = 1.10 (95% CI 0.81-1.49, p = 0.56).
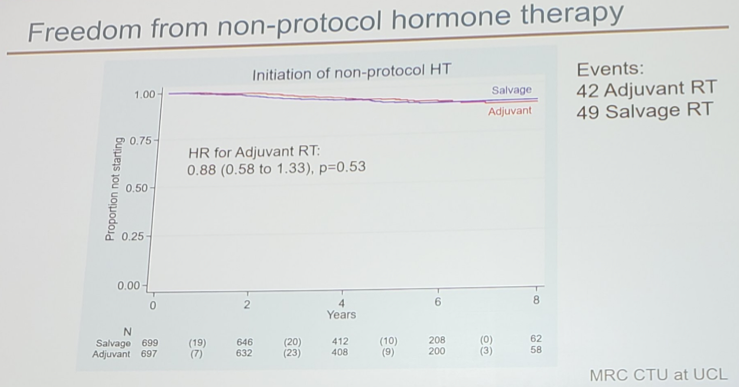
Freedom-from-non-protocol hormone therapy at 5 years was 92% in the adjuvant radiotherapy arm vs 94% in the observation + salvage radiotherapy arm.
Self-reported urinary incontinence was worse at 1 year in the adjuvant radiotherapy patients (5.3%) compared to the observation + salvage radiotherapy patients (2.7%, p = 0.008).
Dr. Parker concluded, noting that the first results from RADICALS-RT trial do not show a benefit for adjuvant radiotherapy after radical prostatectomy in this patient group. Further follow-up is needed to report on long-term outcome measures, including freedom-from-distant metastases. Adjuvant radiotherapy after radical prostatectomy also increases the risk of urinary morbidity. An observation policy with early salvage radiotherapy for PSA failure should be the current standard of care after radical prostatectomy.
Clinical trial identification ISRCTN 40814031
Presented by: Professor Christopher Parker, Consultant Clinical Oncologist BA BM BChir MD FRCR MRCP, The Institute of Cancer Research/Royal Marsden NHS Foundation Trust, Sutton, United Kingdom
Co-Authors: N. Clarke 2, A. Cook 3, H. Kynaston 4, P. Meidahl Petersen 5, W. Cross 6, R.Persad 7, C. Catton 8, J. Logue 9, H. Payne 10, F. Saad 11, K. Brasso 12, H. Lindberg 13, A.Zarkar 14, R. Raman 15, M. Roder 12, C. Heath 16, W. Parulekar 17, M. Parmar 18, M. Sydes 3
1. The Institute of Cancer Research/Royal Marsden NHS Foundation Trust, Sutton, GB
2. The Christie and Salford Royal Hospitals, Manchester, GB
3. Institute of Clinical Trials and Methodology-UCL, London, GB
4. Cardiff School of Medicine, Cardiff, GB
5. Copenhagen University Hospital, Copenhagen, DK
6. St James University Hospital Leeds, GB
7. North Bristol Hospitals, Bristol, GB
8. Princess Margaret Hospital/ University Health Network, Toronto, CA
9. The Christie Hospital, Manchester, GB
10. University College London Hospitals, London, GB
11. Hospital St. Luc du CHUM, Montreal, CA
12. University of Copenhagen, Rigshospitalet, Copenhagen, DK
13. Herlev Hospital, Herlev, DK
14. University Hospital Birmingham, Birmingham, GB
15. Kent Oncology Centre, Canterbury, GB
16. University Hospital Southampton, Southampton, GB
17. Canadian Cancer Trials Group, Kingston, CA
18. MRC Clinical Trials Unit at UCL, London, GB
References:
- Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term follow-up of a randomized clinical trial. J Urol 2019 Mar;181(3):956-962.
- Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: Long-term results of a randomized controlled trial (EORTC trial 22911). Lancet 2012 Dec 8;380(9858):2018-2027.
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct, 2019 in Barcelona, Spain
