Barcelona, Spain (UroToday.com) Single-agent, sequential treatment has been the standard of care in advanced renal cell carcinoma (RCC) for many years – with anti-angiogenic therapy, multi-kinase inhibitors, and anti-PD-1 directed monoclonal antibodies constituting the backbone of therapy. In advanced RCC (after 1 prior line of anti-angiogenic therapy), nivolumab (nivo) shows an overall response rate (ORR) of 25%.1 In previously untreated IMDC intermediate and poor risk advanced RCC, nivolumab plus ipilimumab (nivo + ipi) demonstrated an ORR of 42%.2 The rate of grade 3 and 4 treatment-emergent adverse events (TEAEs) with nivo + ipi is 46% compared to 19% for nivo alone.1,2 Dr. Marc-Oliver Grimm presented results of TITAN-RCC, a large, Phase 2 study investigating a tailored approach starting with nivo alone, but using nivo + ipi as an “immunotherapeutic boost” in non-responders to improve efficacy and reduce TEAEs.
Notable inclusion criteria for TITAN-RCC included patients with metastatic or advanced clear cell RCC, intermediate or high risk by IMDC, measurable disease by RECIST v1.1, KPS https://www.urotoday.com/a1449cd2-b789-4c96-9212-db181662ae6c” width=”9″ height=”14″ /> 70%, and evaluable tumor for PD-L1 expression. Notably, this study included two independent cohort of patients enrolled with 1) no prior therapy (first-line treatment) or 2) one line of prior therapy with a VEGF tyrosine kinase inhibitor (second-line treatment). All patients received nivo 240 mg every 2 weeks with tumor assessment after 4 and 8 doses. Patients with early progressive disease (PD) after 4 doses received two doses of a nivo + ipi boost, after which their response determined whether they received additional doses of nivo + ipi, or returned to nivo maintenance. Patients without PD after 4 doses, were re-assessed after 8 doses; those who had a complete response (CR) or partial response (PR) continued on nivolumab maintenance while those with stable disease (SD) or PD proceeded to nivo + ipi boost. The primary endpoint was ORR with secondary endpoints of progression-free survival (PFS), overall survival (OS), RR after nivo + ipi boosts, and safety.
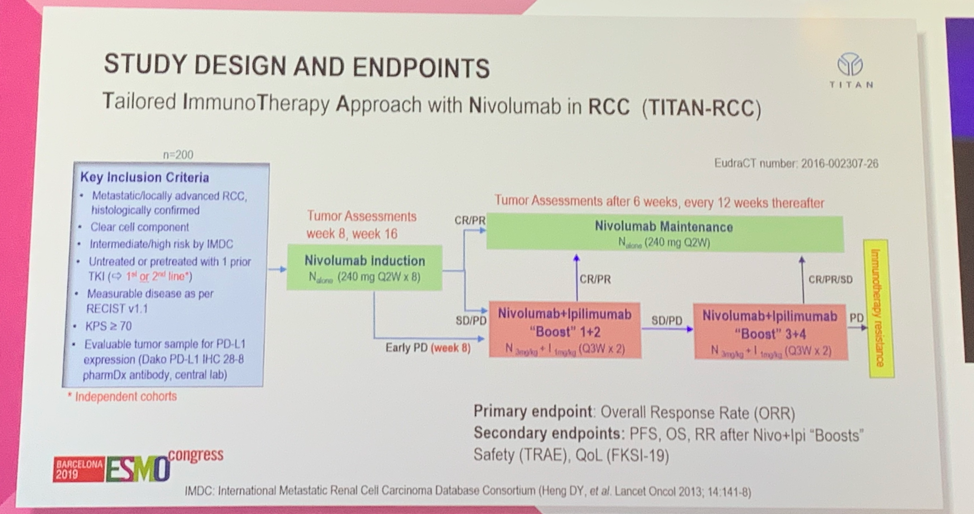
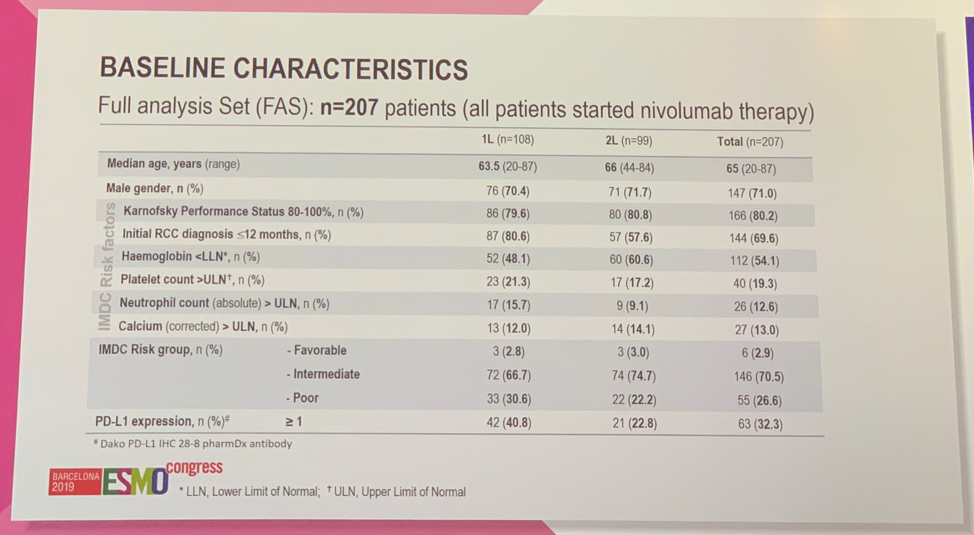
A total of 207 patients were enrolled and treated; 108 in the first-line setting and 99 in the second-line setting. Notable differences between patients treated in the first-line versus second-line setting include the percentage of patients with poor risk IMDC disease (30.6% vs 22.2%) and the percentage of patients with PD-L1 expression https://www.urotoday.com/0bc10757-3a16-4810-914c-1d4d81bf4326″ width=”9″ height=”14″ /> 1 (40.8% vs 22.8%).
The median follow-up at the time of analysis was 36.2 weeks. In the first-line setting, the ORR for patients receiving nivo alone was 28.7% compared to 37.0 % in patients receiving nivo with the nivo + ipi boost. In the second-line setting the ORR was 18.2% among patients receiving nivo alone and 28.3% in those receiving nivo with the nivo + ipi boost. Overall, 64.3% of patients (133/207) received at least one boost cycle.
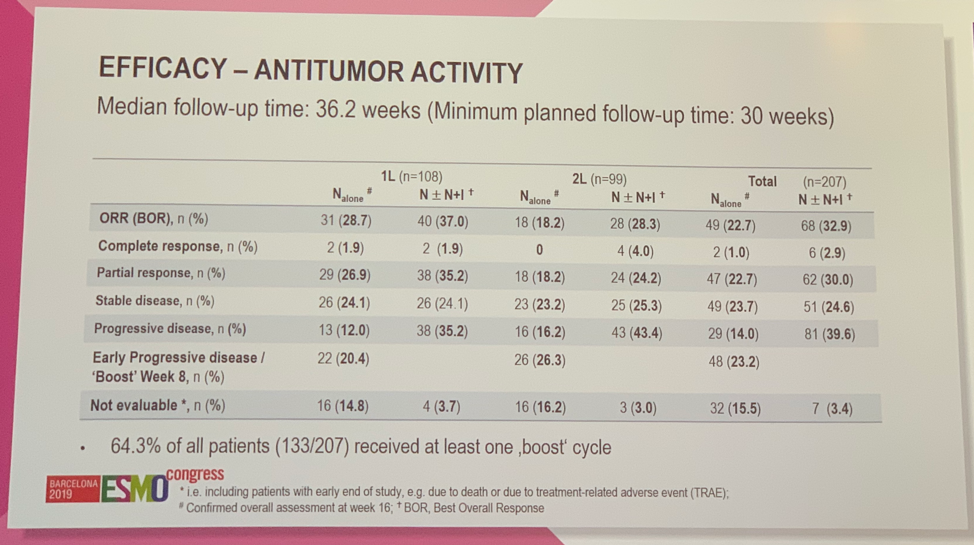
Among patients who received boost cycles, 29.8% of patients in the first-line setting and 38.6% of patients in the second-line setting showed improvement in best response to nivo alone. Median PFS in the first-line setting was 5.5 months for all patients and was not reached for the subset with an initial PR/CR to nivo alone. Median PFS in the second-line setting was 3.7 months for all patients and 16.6 months for patients with an initial PR/CR to nivo alone. Overall survival data is not yet mature.
Safety data for this immunotherapeutic boost strategy in the first-line and second-line setting did not identify any new safety concerns. Any grade 3 or 4 TEAE was observed in 26.9% of patients in the first-line setting and 30.4% of patients in the second line setting. Treatment-related deaths occurred in 1.9% and 1.0% of patients in the first- and second-line setting.
TITAN-RCC is the first study to assess the impact of a tailored approach using ipilimumab as an immunotherapeutic boost to nivolumab monotherapy. This approach improved the ORR from 28.7% to 37.0% in the first-line treatment setting and from 18.2% to 28.3% in the second-line setting. There were no new safety concerns. This study provides further evidence to the added value of ipilimumab in combination with nivolumab in advanced RCC. Further follow-up is ongoing to characterize duration and depth of response, and may support this innovative treatment strategy based on individual response.
Presented by: Marc-Oliver Grimm, MD, Professor and Chairman, Department of Urology, Jena University Hospital, Jena, Germany
References:
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma.
- Motzer RJ, Tannir NM1, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018 Apr 5;378(14):1277-1290.
Written by: Jacob Berchuck, MD, Medical Oncology Fellow at the Dana-Farber Cancer Institute (Twitter: @jberchuck) at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct 2019 in Barcelona, Spain
