Barcelona, Spain (UroToday.com) Although once considered a homologous recombination DNA repair gene, cyclin-dependent kinase 12 (CDK12) is now thought to have a distinct role in maintaining genomic stability. In prostate cancer, inactivating CDK12 mutations, which are found in 6-7% of cases1, lead to gene fusion-induced neoantigens and possibly sensitivity to PD1 inhibitors. At the Prostate Cancer Poster Session at the European Society for Medical Oncology (ESMO) 2019 Congress, Dr. Antonarakis and colleagues presented results of their study assessing outcomes of patients with CDK12 loss-of-function mutations.
This was a retrospective multicenter study to identify advanced prostate cancer patients with loss-of-function CDK12 mutations. The analysis included characterizing these patients’ clinical features and therapeutic outcomes, including sensitivity to poly ADP-ribose polymerase (PARP) and PD1 inhibitors. There were 58 men identified from nine academic centers with at least monoallelic CDK12 alterations, including 28 (48%) men with biallelic inactivation. Tissue for genomics was from primary tumors in 45 cases (77%) and from metastatic sites in 13 cases (23%). All CDK12 mutations were somatic-only. The median age at diagnosis was 60 years (IQR 41–78 years), 71% of men were white, 79% had Gleason sum 9-10 disease, 10% had ductal/intraductal histology, 76% had stage T3/T4 disease, 47% had metastases at diagnosis, and the median PSA was 24 ng/mL (IQR 11.0-98.3 ng/mL).
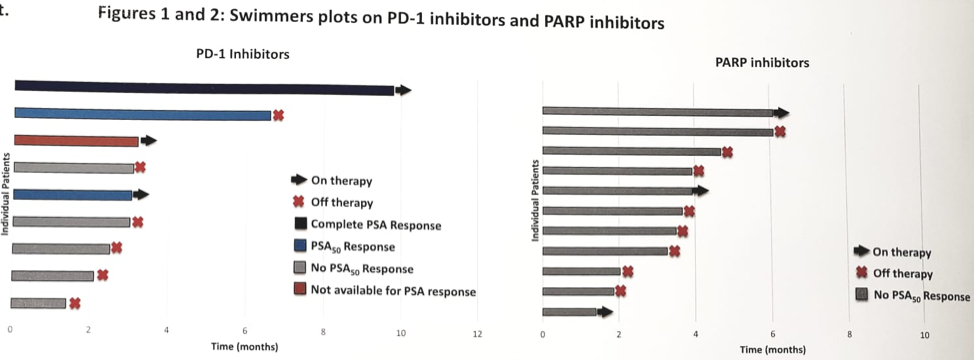
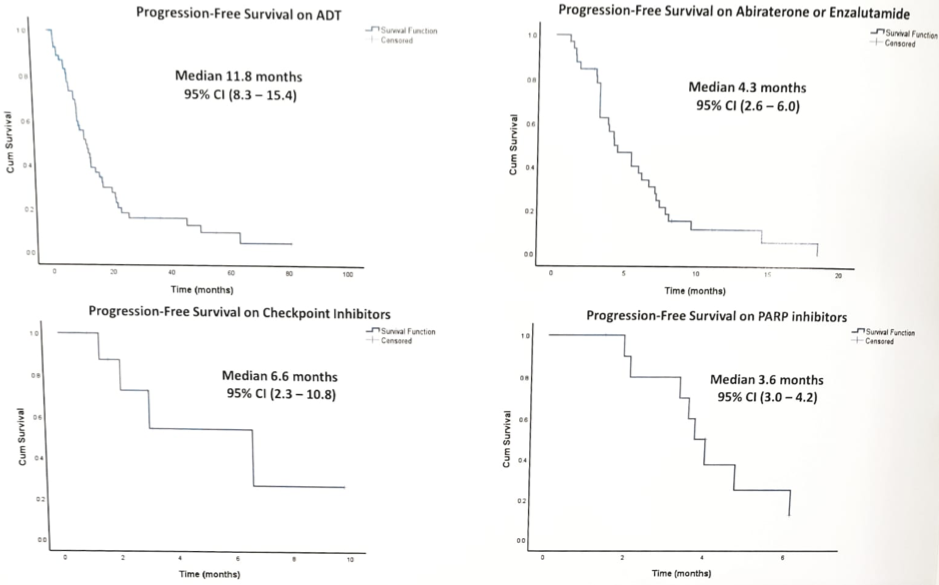
Of those undergoing primary androgen deprivation therapy (ADT) (± abi, ± docetaxel) for advanced disease (n = 54), only 85% had a PSA50 response, with a median progression-free survival (PFS) of 11.8 (95% CI 8.3–15.4) months. The overall survival (OS) from ADT initiation was 40.8 (95% CI 18.7–53) months. Of those receiving first-line abiraterone or enzalutamide for metastatic castrate-resistant prostate cancer (mCRPC) (n = 34), only 47% had a PSA50 response, with median PFS of 4.3 (95% CI 2.6–6.0) months. Of those receiving a taxane agent first for mCRPC (n = 20), only 35% had a PSA50 response, with median PFS of 4.0 (95% CI 2.6–5.3) months. Eleven men received a PARP inhibitor (10 olaparib, one rucaparib), of which none had a PSA50 response, and median PFS was only 3.6 (95% CI 3.0–4.2) months. Eight men received a PD1 inhibitor as 4th to 6th-line mCRPC therapy (five pembrolizumab, three nivolumab): 38% had a PSA50 response, and median PFS was 6.6 (95% CI 2.3–10.8) months.
Dr. Antonarakis concluded with several take-home points for this study assessing outcomes of men with CDK12 mutations:
- CDK12-altered prostate cancer is an aggressive subtype presenting at young age with high Gleason grade and often with de novo M1 disease at diagnosis
- Outcomes to hormonal and taxane therapies are poor, and PARP inhibitors are also ineffective
- A proportion of these patients respond favorably to PD1 inhibitors, implicating CDK12 deficiency in immunotherapy responsiveness.
Presented by: Emmanuel S. Antonarakis, MB BCh, Associate Professor of Oncology and Urology, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore, Maryland
Co-Authors: Isaacsson Velho1, N. Agarwal2, V. Sacristan Santos2, B. Maughan2, R. Pili3, N. Adra3, C. Sternberg4, P. Vlachostergios4, S. Tagawa5, A. Bryce6, A. Mcnatty6, Z. Reichert7, R. Dreicer8, O. Sartor9, T. Lotan1, M. Hussain10
1. Johns Hopkins University School of Medicine, Baltimore, US
2. Huntsman Cancer Institute, Salt Lake City, US
3. Indiana University School of Medicine, Indianapolis, US
4. Weill Cornell Medicine, New York, US
5. NewYork-Presbyterian Hospital/ Weill Cornell Medical Center, New York, US
6. Mayo Clinic/Scottsdale, Scottsdale, US
7. University of Michigan, Ann Arbor, US
8. University of Virginia Cancer Center, Charlottesville, US
9. Tulane University, New Orleans, US
10. Northwestern University Feinberg School of Medicine, Chicago, US
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct, 2019 in Barcelona, Spain
Reference:
