Barcelona, Spain (UroToday.com) Although once considered a homologous recombination DNA repair gene, CDK12 is now thought to have a distinct role in maintaining genomic stability. In prostate cancer, inactivating CDK12 mutations lead to gene fusion-induced neoantigens, which may result in sensitivity to immunotherapeutic treatment strategy. The authors of this abstract conducted a retrospective, multicenter study to identify advanced prostate cancer patients with loss-of-function CDK12 alterations. They then characterized the clinical features and therapeutic outcomes of these patients, including sensitivity to PARP and PD1 inhibitors.
Investigators from 9 academic centers identified 58 men with either monoallelic (52%) or biallelic (48%) CDK12 alterations. Genomic analysis was performed on primary tumors in 77% of cases and metastatic sites in the remaining 23%. All alterations were somatic. The median age of patients was 60 and the majority (71%) were white. These patients were enriched for aggressive characteristics including Gleason sum 9-10 (79%), T3/T4 disease (76%), and median PSA at diagnosis of 24 ng/ml. Among the 54 patients who received primary ADT for advanced disease, only 85% of those had a greater than 50% drop in PSA (PSA50) was a median progression-free survival (PFS) of 11.8 months and overall survival (OS) of 40.8 months (from the time of ADT initiation). Of the 34 patients receiving abiraterone or enzalutamide in the first-line mCRPC setting, only 47% had a PSA50 response with a median PFS of 4.0 months. Disappointingly, of 11 men who received a PARP inhibitor (10 olaparib; 1 rucaparib), none had a PSA50 response with a median PFS of 3.6 months. In contrast, 8 men received a PD1 inhibitor as 4th line or later mCRPC therapy (5 pembrolizumab; 3 nivolumab) and 38% had a PSA50 response with a median PFS of 6.6 months.
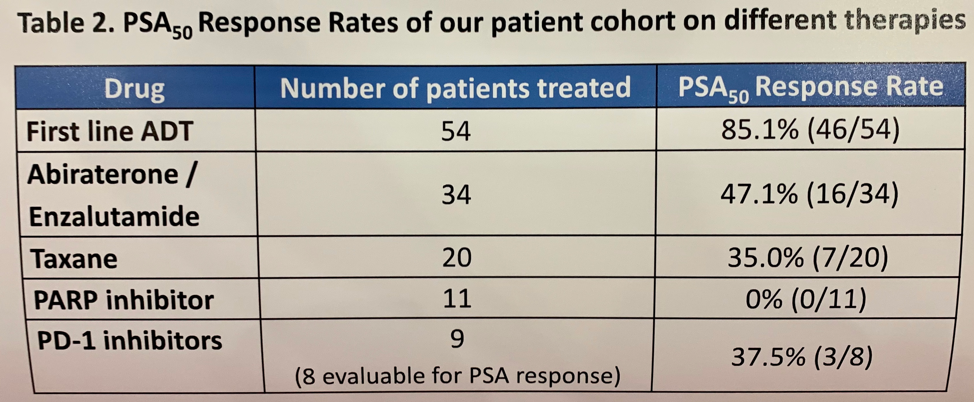
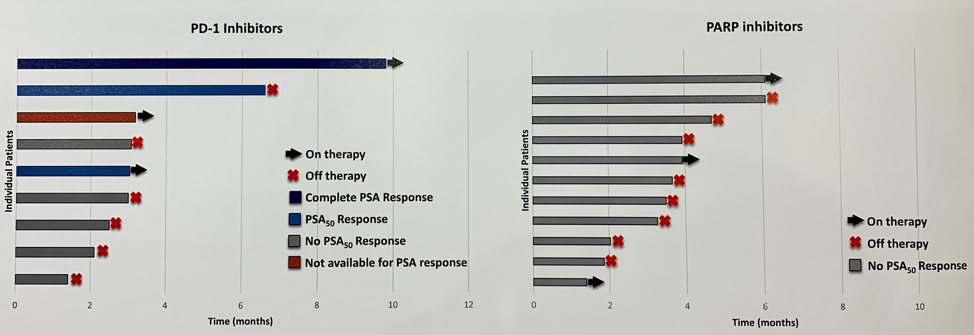
The authors conclude that CDK12-altered prostate cancer is an aggressive subtype that presents at young age, with high Gleason grade, and often de novo metastatic disease at diagnosis. Response rates and duration of response to hormonal therapies, chemotherapy, and PARP inhibitors are poor. A significant proportion of these patients (38%), however, responded to PD-1 inhibitors, though duration of response is relatively short (6.6 months). This study provides compelling data to support further investigation into immunotherapy treatment strategies for patients with CKD12-altered prostate cancer. The ongoing phase 2 IMPACT study (NCT03570619) aims to determine the efficacy of nivolumab and ipilimumab combination therapy followed by nivolumab monotherapy in patients with metastatic prostate cancer harboring loss of CDK12 function.
Presented By: Emmanuel Antonarakis, M.B.B.Ch., Professor of Oncology at Johns Hopkins University School of Medicine
Written by: Jacob Berchuck, MD, Medical Oncology Fellow at the Dana-Farber Cancer Institute, Twitter: @jberchuck at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct 2019 in Barcelona, Spain
