Barcelona, Spain (UroToday.com) Enfortumab vedotin is an antibody-drug conjugate comprised of the nectin-4 antibody enfortumab coupled to the microtubule disrupting agent monomethyl auristatin E (MMAE). In preclinical models, MMAE-based antibody drug conjugates were shown to be capable of inducing immunogenic cell death, thereby augmenting the anti-tumor immune response.1 Furthermore, a recently published single-arm phase II study demonstrated an impressive 44% objective response rate with enfortumab vedotin monotherapy among a heavily pre-treated cohort of advanced urothelial carcinoma patients who had progressed on prior immune checkpoint blockade as well as on prior platinum-based chemotherapy.2 On the basis of these encouraging clinical data and the strong biologic rationale for combining with immune checkpoint blockade, investigators conducted EV-103, a phase 1b dose-escalation and expansion study of the antibody drug conjugate enfortumab vedotin in combination with the PD-1 antibody pembrolizumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma.
Dr. Hoimes presented data on cohort A, which consists of first-line cisplatin-ineligible patients. A total of 45 patients received enfortumab vedotin (1.25 mg/kg days 1 and 8 of every 3-week cycle) in combination with pembrolizumab (200mg every 3 weeks). The prespecified primary endpoints were safety and tolerability, and key secondary endpoints included objective response rate, duration of response, and overall survival.
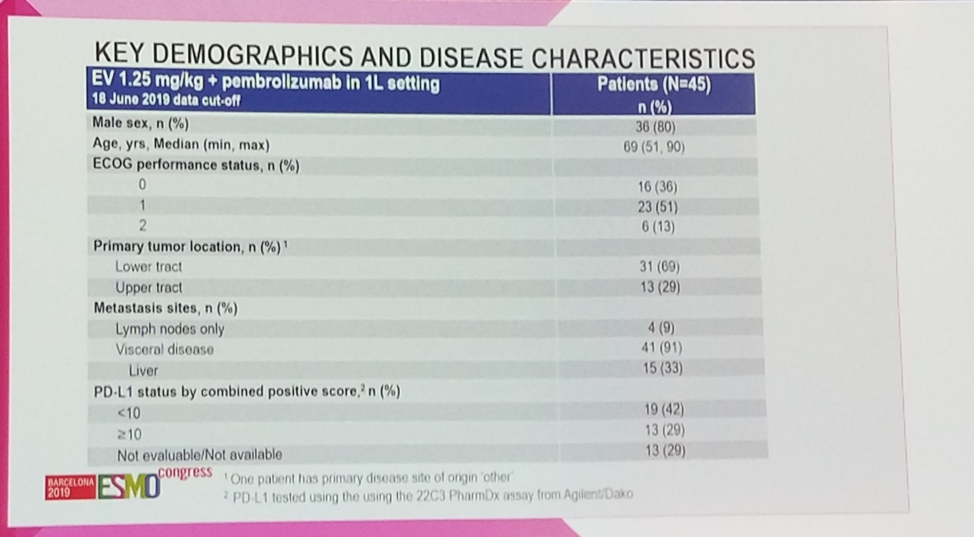
The 45 patients enrolled on this study are fairly representative of the advanced bladder cancer population globally, with an 80% male predominance and a median age of 69. Of note, 29% (13/45) of patients had an upper-track primary, while 69% (31/45) had a bladder primary. Most importabtly, 91% (41/45) of the cohort had visceral metastases, including 33% (15/45) with liver metastases, a subgroup which has been exceedingly challenging to treat with immunotherapy or chemotherapy.
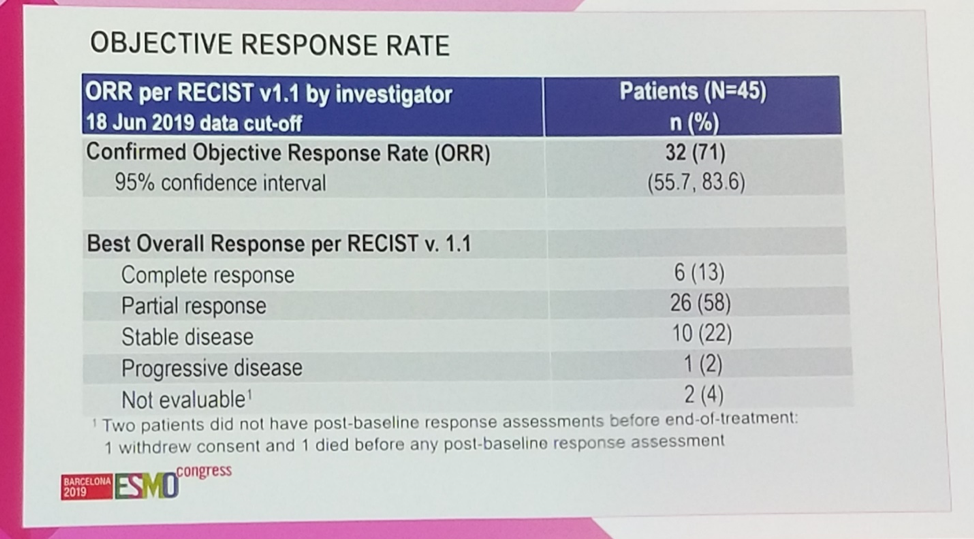
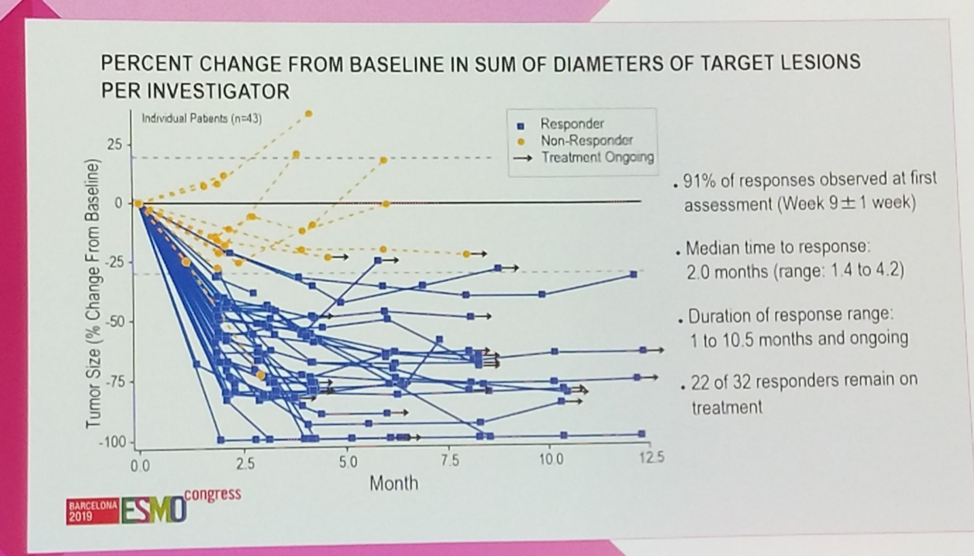
Investigators confirmed a striking 71% (32/45) objective response rate (95% confidence interval 55.7 – 83.6), with 13% (6/45) achieving a complete response, and another 58% (26/45) achieving a partial response. An additional 22% (10/45) had stable disease, for a remarkable disease control rate of 93%.
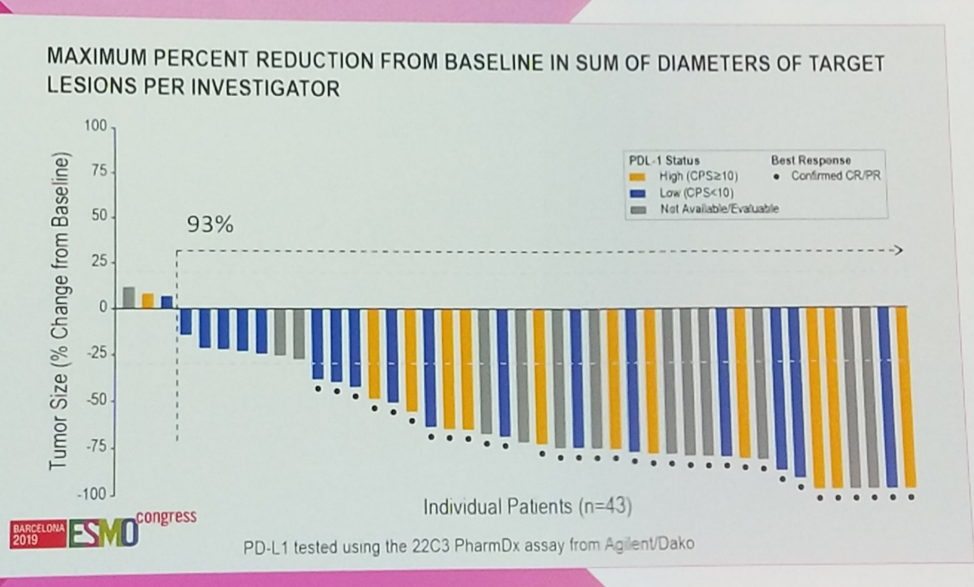
Of note, responses were observed irrespective of PD-L1 expression as measured by the combined positive score.
Encouragingly, responses were observed at a median of 2.0 months from the time of treatment initiation, and response duration ranged from 1.0 to 10.5 months. As of last follow-up, 69% (22/32) of responses were ongoing.
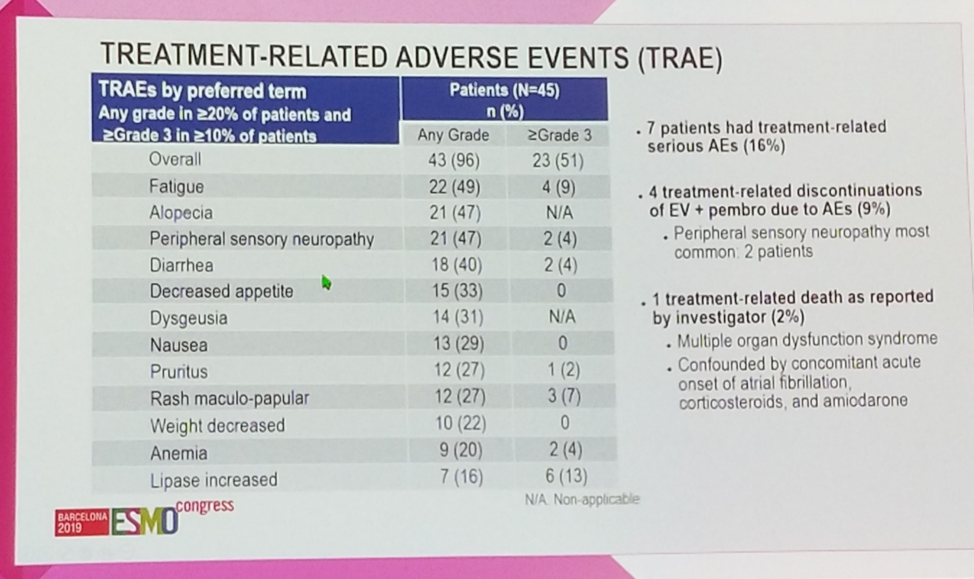
In terms of safety, 51% (23/45) of patients experienced a grade 3 or grade 4 adverse event. Fatigue, alopecia, neuropathy, and diarrhea were the most common toxicities (40-49%), and 13% (6/45) of patients experienced grade ≥3 lipase elevation. A single patient (2%) died of multiorgan dysfunction in the context of acute kidney injury and atrial fibrillation with rapid ventricular response.
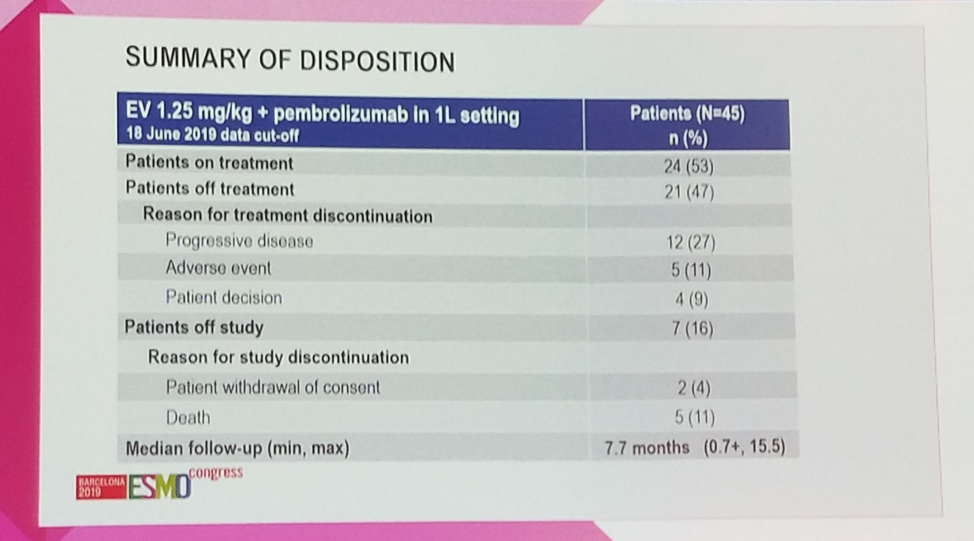
At a median follow-up of 7.7 months, the majority of patients remain on treatment, with 47% (21/45) discontinuing, most commonly for progression of disease.
In summary, the combination of the nectin-4/MMAE- based antibody-drug conjugate enfortumab vedotin and the PD-1 antibody pembrolizumab achieved a remarkable 71% objective response rate in a modest-sized phase 1b expansion cohort of first-line cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer, many of whom exhibited adverse features such as liver metastases. While this efficacy signal is very encouraging, larger trials may be needed to more completely characterize the efficacy of this combination. It must also be acknowledged that treatment options are limited in this disease setting, particularly as single anti-PD-1 or anti-PD-L1 immunotherapy has been restricted to patients with PD-L1-high tumors within the first-line setting, and outcomes with carboplatin-based chemotherapy are poor. Future work will hopefully elucidate the biologic mechanisms by which antibody-drug conjugates such as enfortumab vedotin are able to induce immunogenic cell death to better understand how best to combine with checkpoint blockade immunotherapy in the clinic.
Presented By: Christopher J. Hoimes, DO, Case Comprehensive Cancer Center, Cleveland, US
Written by: Michael Lattanzi, MD, Medical Oncology Fellow, Memorial Sloan Kettering Cancer Center Twitter: @MikeLattanzi at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct 2019 in Barcelona, Spain
References:
- Cao A, Heiser R, Law CL, Gardai SJ. Auristatin-based antibody drug conjugates activate multiple ER stress response pathways resulting in immunogenic cell death and amplified T-cell responses. Cancer Research. 2016; 76(14) 4914-4914.
- Rosenberg JE, O’Donnell PH, Balar AV, McGregor BA, Heath EI, Yu EY, Galsky MD, Hahn NM, Gartner EM, Pinelli JM, Liang SY. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. Journal of Clinical Oncology. 2019 Jul 29:JCO-19.
