Barcelona, Spain (UroToday.com) In this session, Dr. Cristina Suarez discussed the prior two sessions including 1) Primary efficacy analysis results from the SORCE trial: Adjuvant sorafenib for renal cell carcinoma (RCC) at intermediate or high risk of relapse: an international, randomised double-blind Phase 3 trial, and 2) results from the Tailored ImmunoTherapy Approach with Nivolumab in Advanced Renal Cell Carcinoma (TITAN-RCC) trial.
Surgical resection is the standard of care for patients with organ-confined RCC and often results in cure. However, 30-40% of patients with high-risk features will experience disease recurrence. Inactivation of VHL underlies the development of a majority of clear cell RCC and increases VEGF expression. Dr. Suarez highlighted the four large, randomized Phase 3 clinical trials (S-TRAC, ASSURE, PROTECT, and ATLAS) that evaluated adjuvant VEGF tyrosine kinase inhibitor (TKI) therapy in patients with resected RCC. The SORCE trial is now the fifth trial to report out in this space. Eligible patients had intermediate or high risk resected RCC. 1,711 patients were randomized 2:3:3 to placebo (Arm A), 1 year of sorafenib followed by 2 years of placebo (Arm B), and 3 years of sorafenib (Arm C).
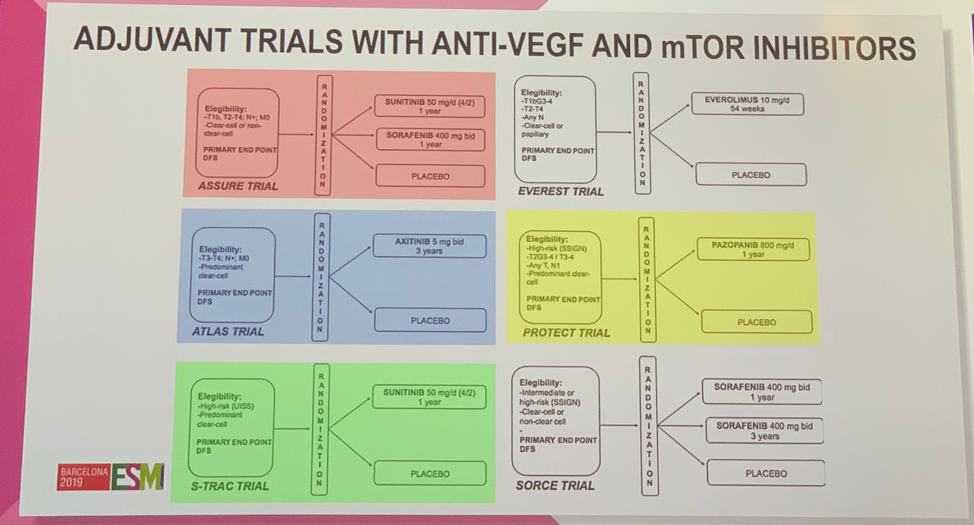
Of these five studies, only S-TRAC met the primary endpoint of improvement in disease-free survival (DFS). None of these studies demonstrated an improvement in overall survival (OS).
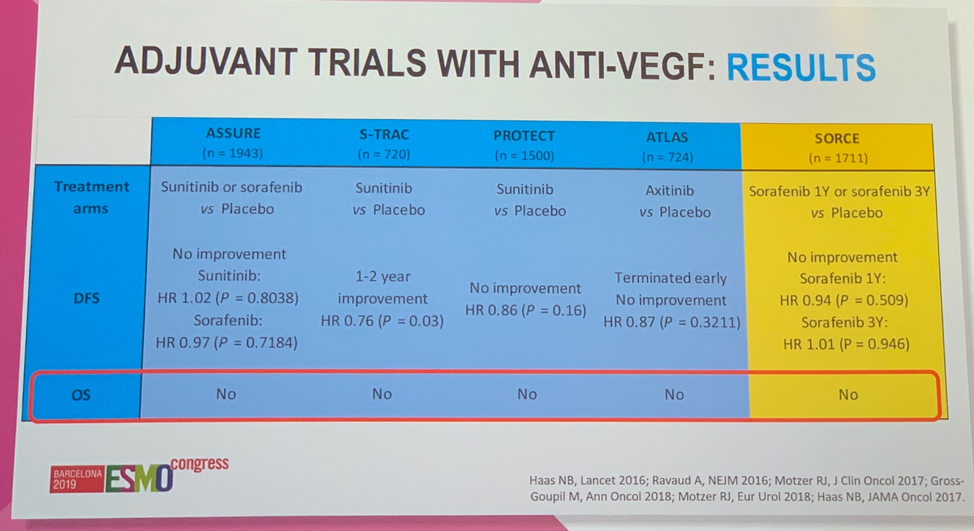
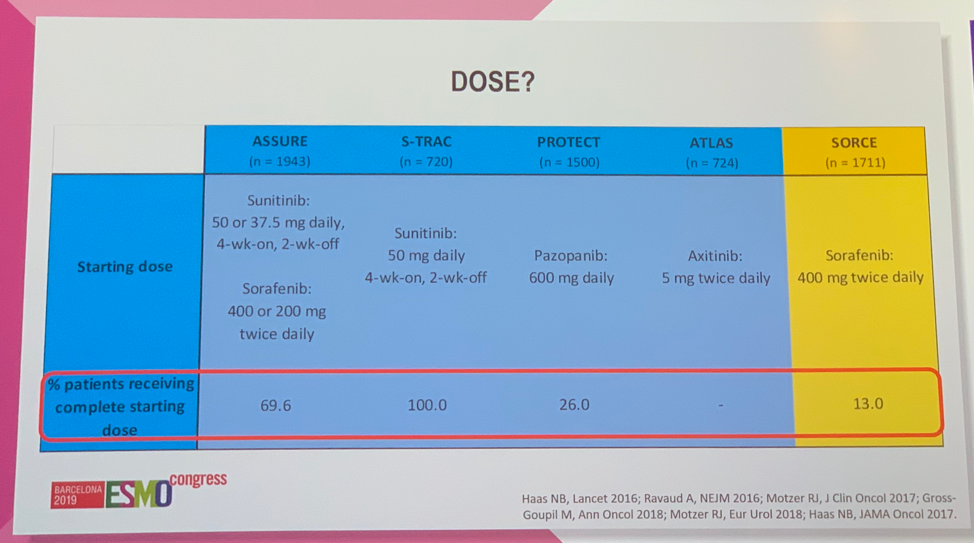
Further, Dr. Suarez showed a meta-analysis of these trials that found no benefit in DFS or OS.1 She then sought to address some of the factors that may have contributed to these trials being unsuccessful. Across the trials, there was a low percentage of patients receiving treatment with the intended full dose, except for S-TRAC – the one trial that was positive for improved DFS.
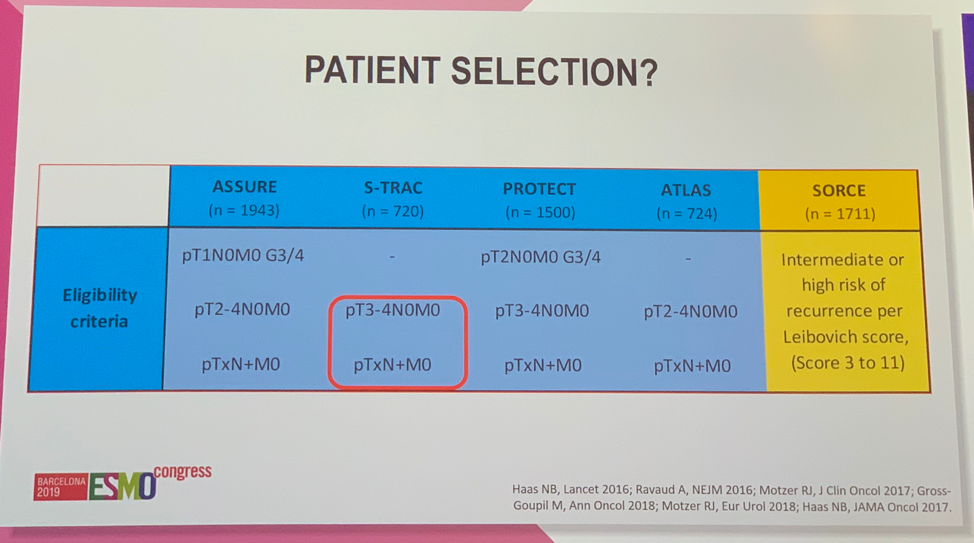
Inclusion criteria and patient selection was another factor Dr. Suarez highlighted as possibly contributing to the observed results. S-TRAC was the only trial to enroll exclusively high-risk patients.
Finally, Dr. Suarez hypothesized that the mechanism of action of VEGF TKIs may make them less likely to be effective in the adjuvant setting compared to the metastatic setting. VEGF TKIs work by blocking neovascularization and vascular trimming, resulting in intratumor hypoxia, which prevents tumor growth. Compared to overt metastases, which are highly vascular, micrometastases, the type of disease present in the adjuvant setting are less vascular, thus less responsive to anti-angiogenic therapy. She concluded that SORCE is now the fifth Phase 3 clinical trial with a VEGF TKI in the adjuvant setting demonstrating no improvement in OS for RCC and the fourth to show no improvement in DFS. Thus, given the lack of efficacy and significant toxicity associated with this therapy, adjuvant therapy for RCC should only be considered as part of a clinical trial. The prospect of improved outcomes in the adjuvant RCC setting now rests on immunotherapy where many trials are ongoing.
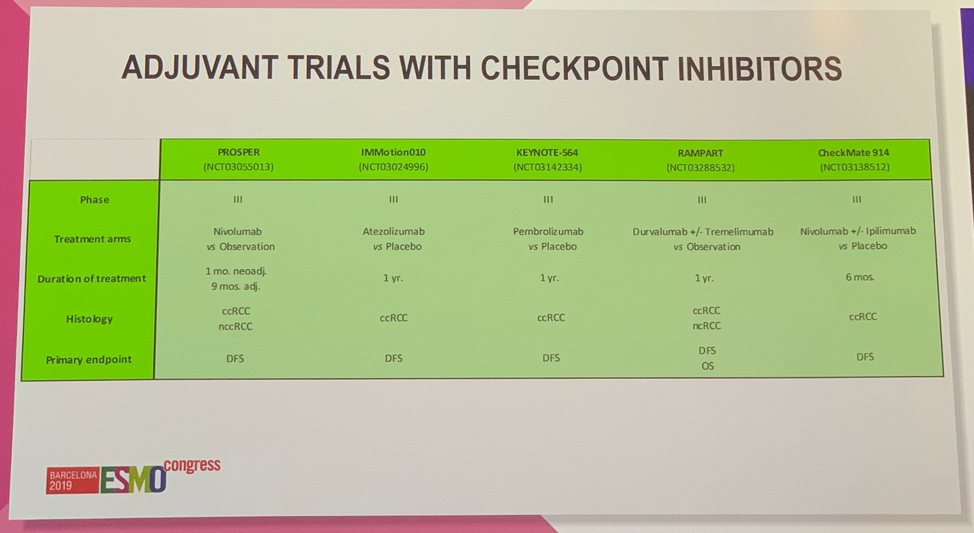
Dr. Suarez then moved on to discuss the results from the Tailored ImmunoTherapy Approach with Nivolumab in advanced Renal Cell Carcinoma (TITAN-RCC) trial, the first study to assess the impact of a tailored approach using ipilimumab as an immunotherapeutic boost to nivolumab therapy.
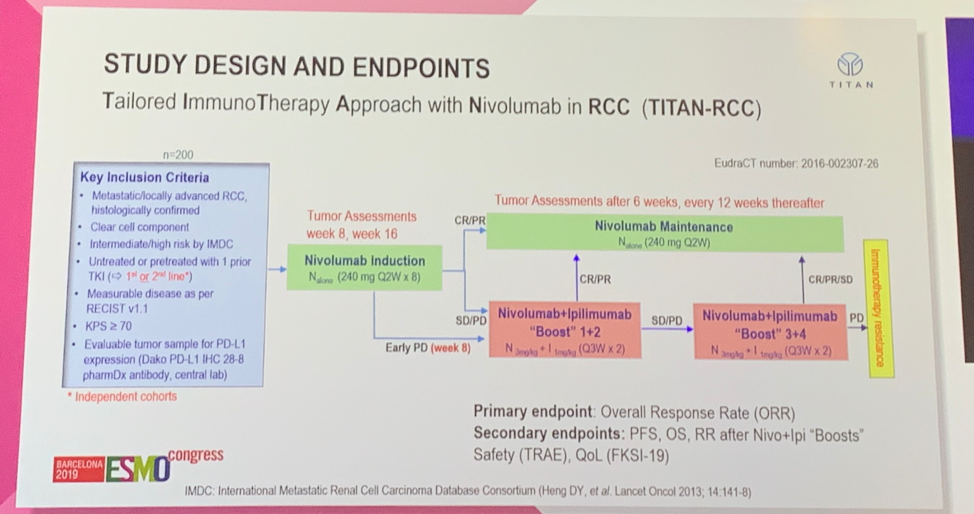
Dr. Suarez first summarized the primary finding from this study that approximately 10% of patients who did not respond to nivolumab alone had a subsequent response, or were “rescued”, after receipt of nivolumab plus ipilimumab. This finding was true in the first-line and the second-line treatment setting following VEGF TKI. She argued that these are distinct patient populations with different pros and cons. In the first-line setting nivolumab plus ipilimumab is a standard of care option. The benefit of the TITAN-RCC treatment approach in this setting would be reducing the number of patients receiving ipilimumab and thus reducing the number of adverse events. The downside would be that some patients may miss the opportunity to receive ipilimumab (33% in TITAN-RCC), and OS data is immature, so it is not clear how long-term survival with this approach compares to upfront nivolumab plus ipilimumab. Dr. Suarez argued that in the second-line setting, where only nivolumab monotherapy is approved, there is a significant upside to this approach, whereby adding ipilimumab presents the opportunity to rescue patients after progression. The only downside would be a potential increase in toxicity with the possibility that there is no improvement in long-term survival as the impact of this approach on OS is not yet known. Dr. Suarez thus concluded that immunotherapeutic boost is a promising strategy in the second-line setting, though less so as first-line therapy.
Clinical Trial Information:
NCT00492258
NCT02917772
Presented by: Cristina Suarez, MD, Ph.D., Medical Oncologist at the Vall d´Hebron University Hospital, Hospital in Barcelona, Spain
References:
Written by: Jacob Berchuck, MD, Medical Oncology Fellow at the Dana-Farber Cancer Institute (Twitter: @jberchuck) at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct 2019 in Barcelona, Spain
