Barcelona, Spain (UroToday.com) Dr. Cristina Suarez provided the invited discussion for the two kidney cancer studies presented, SORCE and TITAN-RCC. Dr. Suarez notes that adjuvant therapy in RCC is needed in that 30-40% of patients with high-risk features (high nuclear grade, locally advanced stage, and/or regional lymph node involvement) will experience disease recurrence. Indeed, antiangiogenics have been tested in the adjuvant setting in five phase III clinical trials, including sunitinib (two trials), axitinib, everolimus, pazopanib, and now sorafenib – the sixth trial (SORCE) presented today. All of these trials have failed to provide an OS benefit in the adjuvant setting. Furthermore, only one trial, S-TRAC,1 has noted an improvement in DFS (HR 0.76, p=0.03) with adjuvant sunitinib. Aside from SORCE (21.6%), all trials have had grade 3-4 adverse events >60%.
Dr. Suarez asks, why have all of these trials failed? Dose? Patient selection? Mechanism of action? Perhaps because of the degree of adverse events, very few people have been able to complete the trial at the starting dose, including only 13% in the SORCE trial. A subgroup analysis of PROTECT showed an improvement in DFS in the 800 mg group, compared to the 600 mg group (31% vs 14%).2 Thus, inferior dosing may lead to worse results. Perhaps we haven’t been selecting the highest risk patients who may derive the greatest benefit, however a subgroup analysis of high risk patients in ASSURE3 and ATLAS4 demonstrated no difference when stratifying by risk. With regards to mechanism of action, antiangiogenics work by (i) blocking neovascularization (angiogenesis), (ii) vascular trimming, (iii) intratumor hypoxia, and (iv) blocking of tumor expansion, a mostly cytostatic effect. Dr. Suarez notes that in overt metastasis, these areas are highly vascular and antiangiogenics can trim vessels, however micrometastases (which is what is being “treated” in adjuvant therapy) are essentially avascular and cannot trim vessels.
We eagerly await the completion of the adjuvant trials with checkpoint inhibitors:
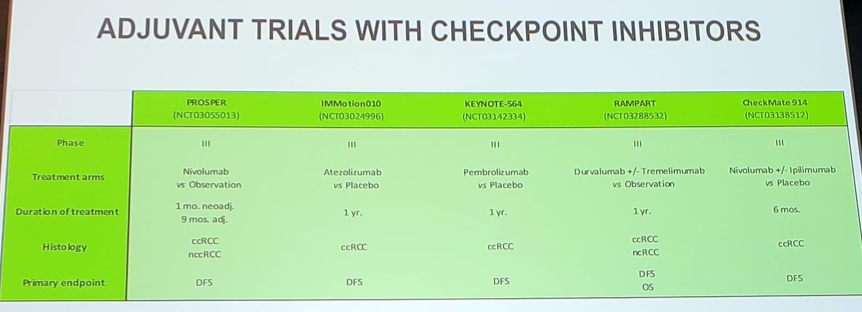
Dr. Suarez then discussed the TITAN-RCC study, which assessed nivolumab therapy followed by nivolumab + ipilimumab boosts in both the first and second-line setting. The study was powered to find an ORR of 40% compared to the zero-hypothesis ORR of 25%; however, Dr. Suarez question whether a unique objective fits for both the first and second line setting. Combining all patients, the ORR for nivolumab monotherapy was 22.7% and 32.9% after the nivolumab + ipilimumab boost, suggesting that 10% of patients can be rescued who initially do not do well with nivolumab monotherapy. In the first line setting, the pros of this study design are that we may be able to reduce adverse events by adding ipilimumab later, whereas the cons are that some patients may miss the opportunity to receive ipilimumab. In the second-line setting, the pros are that this study provides the opportunity of rescuing patients that progress on nivolumab monotherapy, whereas there really are no cons other than possibly adding toxicity with no efficacy.
The first-line results of TITAN-RCC demonstrating an ORR of 37% after nivolumab + ipilimumab boost is comparable to the 37-59% ORR reported by previous trials. Furthermore, the demographics are similar to those of CheckMate 214 with regards to intermediate/poor prognosis break down. The CA209-8Y8 will further clarify timing of nivolumab and ipilimumab in the first line setting: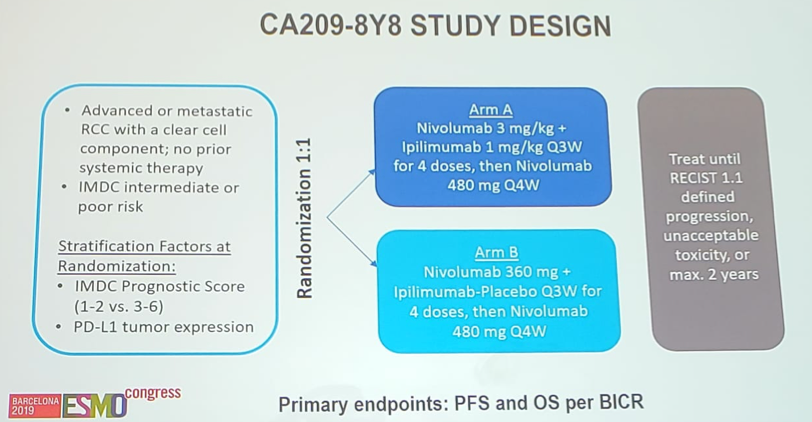
The second line TITAN-RCC results demonstrate a 28.3% ORR after nivolumab + ipilimumab boost, suggesting that even in the second line, 10% of patients can be rescued. This ORR is better than both the METEOR (ORR 17%) and CheckMate-025 (25%) second line trials. However, overall, Dr. Suarez notes that this strategy may not be fit for all patients, as 33% of patients did not receive the nivolumab + ipilimumab boost, thus never experiencing a potential benefit of ipilimumab.
Dr. Suarez concluded her discussion with several summary points:
- Ipilimumab boost could rescue ~10% of patients
- Not all candidates could receive the ipilimumab boost, specifically only 77% in the first-line setting
- This may be a decent strategy for the second line, but does not appear to be as efficacious in the first-line
- PFS and OS data are immature
Presented by: Cristina Suarez, MD, PhD, Physician-Scientist, VHIO´s Genitourinary, CNS Tumors, Sarcoma & Cancer of Unknown Primary Site Group, headed by Joan Carles, and Medical Oncologist at the Vall d´Hebron University Hospital (HUVH) Barcelona, Spain
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct 2019 in Barcelona, Spain
References:
- Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med 2016;375(23):2246-2254.
- Motzer RJ, Haas NB, Donskov F, et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with locally advanced renal cell carcinoma (RCC) (PROTECT). J Clin Oncol 2017;35(35):3916-3923.
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016;387(10032):2008-2016.
- Gross-Goupil M, Kwon TG, Eto M, et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Ann Oncol 2018 Dec 1;29(12):2371-2378.
