Barcelona, Spain (UroToday.com) Professor Jan Oldenburg from Oslo, Norway provided a discussion of Dr. Silke Gillessen’s update of the International Germ Cell Cancer Collaborative Group (IGCCCG) NSGCT risk groups at ESMO 2019 annual congress. As Dr. Oldenburg notes, the original IGCCCG prognostic factor-based staging system was published more than two decades ago in 1997.1 This classification system has been very pragmatic, easy to use, but also with considerable heterogeneity within groups, particularly the intermediate risk group.
Several studies have assessed the prognosis of IGCCC groups based on chemotherapy dose and regimen. Recently, the International Global Germ Cell Tumor Collaborative Group (G3) investigated the prognostic impact of different tumor marker levels prior to first line chemotherapy within the intermediate risk group.2 Among 634 patients with a median follow-up of 9.0 years (IQR 14 years), patients receiving first line treatment with platinum based chemotherapy had a 5-year OS rate of 87%. The stratification of patients according to AFP levels revealed a correlation between AFP levels and outcome: 5-year OS rates of:
- 88% for AFP levels <1,000 IU/ml (n = 303)
- 89% for 1,000 to 2,000 IU/ml (n = 82)
- 87% for >2,000 to 6,000 IU/ml (n = 121)
- 82% for >6,000 IU/ml (n = 57)
Different HCG levels prior to chemotherapy were not associated with outcome. In multivariable analysis AFP levels >6,000 IU/ml (p = 0.023; HR 2.26) or >1,982 IU/ml (p = 0.031; HR 1.72), and LDH levels >3 UNL (p < 0.001; HR 2.62) were independent prognosticators for OS. Thus, there is a high level of heterogeneity in the intermediate risk group.
A recent review of survival rates stratified by the IGCCC prognostic groups demonstrates that the IGCCC update has improved OS for all prognostic groups, in addition to tight confidence intervals:
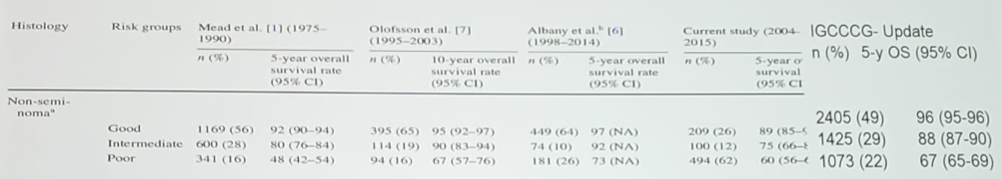
Dr. Oldenburg states we are doing better, but why? In his opinion, practice makes perfect and lists the following points as to why outcomes are improved, despite utilization of the same chemotherapy since the implementation of cisplatin by Dr. Einhorn in the 1970’s:
- Accurate and rapid diagnosis, as well as pathologic characterization of risk
- Risk adapted chemotherapy for metastatic disease on the basis of a uniform prognostic classification (IGCCCG)
- Understanding nuances and behavior of elevated tumor markers and equivocal imaging
- Surgical advances: management of larger primaries, nerve-sparing RPLND, ERAS protocols, and management of post-chemotherapy residual disease
- Chemotherapy: standardized delivery to achieve near 100% dose intensity, control of nausea and vomiting, moving chemotherapy deliver primarily to the outpatient setting, appropriate use of growth factors, and limited use of vascular devices
- Recognition and management of thrombotic risk
- Less intensive imaging and follow-up for early stage and post-primary treatment
- Clinical and biologic characterization of risk for long-term adverse effects
Although Dr. Oldenburg acknowledges that it may not be practical, certainly the advent of the biomarker miR-371a-3p in the new IGCCCG prognostic risk groups would be great. As has recently been shown, miR-371a-3p positive patients have worse PFS and OS.3 Finally, Dr. Oldenburg notes that with the new classification it is important to continue to delineate the exceptional prognosis patients (particularly in the good and intermediate group), as these may be patients that can be targeted for decreased treatment intensification (don’t compare “apples to pears”).
Dr. Oldenburg concluded his presentation with several summary points:
- Relevant ground-breaking or better: this is ground providing work by the EORTC and Gillessen team
- The LDH cut-off of 2.5 ULN, age, and the presence of lung metastases: improves prognostication
- The IGCCCG Update calculator improves accuracy and discrimination
- Be sure to not compare apples to pears – further patient delineation, particularly those with excellent prognosis in the good and intermediate risk groups, is required
Presented by: Jan Oldenburg, MD, PhD, Clinical Oncologist at the University of Oslo, Oslo, Norway
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct 2019 in Barcelona, Spain
References:
- International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: A prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol 1997 Feb;15(2):594-603.
- Seidel C, Daugaard G, Tryakin A, et al. The prognostic impact of different tumor marker levels in nonseminomatous germ cell tumor patients with intermediate prognosis: A registry of the International Global Germ Cell Tumor Collaborative Group (G3). Urol Oncol 2019 Sep 4 [Epub ahead of print].
- Mego M, van Agthoven T, Gronesova P, et al. Clinical utility of plasma miR-371-3p in germ cell tumors. J Cell Mol Med 2019 Feb;23(2):1128-1136.
