Barcelona, Spain (UroToday.com) Effective therapies for patients with treatment-refractory metastatic castration-resistant prostate cancer (mCRPC) are an important unmet medical need. Niraparib is a highly selective poly ADP-ribose polymerase (PARP) inhibitor of PARP-1 and PARP-2 DNA-repair polymerases, proving effective for patients with mCRPC and DNA repair defects, initially presented at the 2019 ASCO meeting1. The Phase II GALAHAD study is being conducted to assess the efficacy and safety of niraparib in patients with mCRPC and DNA repair defects who have progressed on androgen receptor (AR) targeted therapy and taxane-based chemotherapy. At the European Society for Medical Oncology (ESMO) 2019 Congress Prostate Cancer Poster Session, Dr. Matthew Smith presented results from a pre-specified interim analysis.
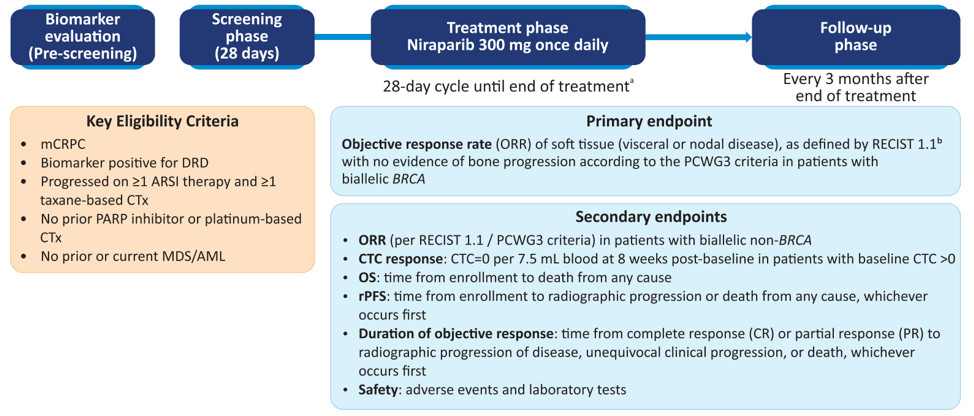
GALAHAD is an ongoing open-label Phase II study assessing niraparib (300 mg daily) in patients with mCRPC and DNA repair defects with disease progression on taxane and androgen receptor-targeted therapy. DNA repair defect status was evaluated by a plasma or tissue-based test and defined as having biallelic alterations in BRCA1/2 (BRCA), ATM, FANCA, PALB2, CHEK2, BRIP1, or HDAC2. The primary endpoint was objective response rate (ORR) by RECIST 1.1 with no evidence of bone progression as per the PCWG3 criteria. Composite response rate (CRR) was defined as ORR, conversion of circulating tumor cells to < 5/7.5 mL blood, or ≥ 50% decline in prostate-specific antigen (PSA).
As of May 23, 2019, 223 patients were screened for eligibility, 165 patients were enrolled, of whom 81 had biallelic DNA repair defects (46 BRCA and 35 non-BRCA) and had a minimum of 16 weeks of follow up. Of the patients with biallelic DNA repair defects, 51 (63%) had measurable disease at baseline (29 BRCA and 22 non-BRCA) and 47% of patients had visceral metastases. The mean age was 68.2 years (SD 7.6 years) and 30% had ≥4 prior therapies for prostate cancer. Furthermore, 99% of patients had received prior docetaxel, and 40% previous cabazitaxel. Median follow-up in BRCA patients was 7.3 months and 6.4 months for non-BRCA. In BRCA patients, ORR was 41% and CRR was 63%, with a median duration of objective response of 5.5 months (range: 3.5–9.2 months). Median radiographic progression-free survival (rPFS) and OS in BRCA patients were 8.2 and 12.6 months, respectively. In non-BRCA, objective response was noted in only 9% of patients and CRR was 17%, with durations of objective response of 3.8 and 6.5 months, respectively. The median rPFS for non-BRCA patients was 5.3 months and OS was 14.0 months. Among patients with measurable disease, 38% of BRCA patients had a partial response, compared to only 9% of non-BRCA patients. Among BRCA patients, 50% had a ≥ 50% decline in PSA, compared to 3% of non-BRCA patients.
Maximum change in PSA on niraparib:
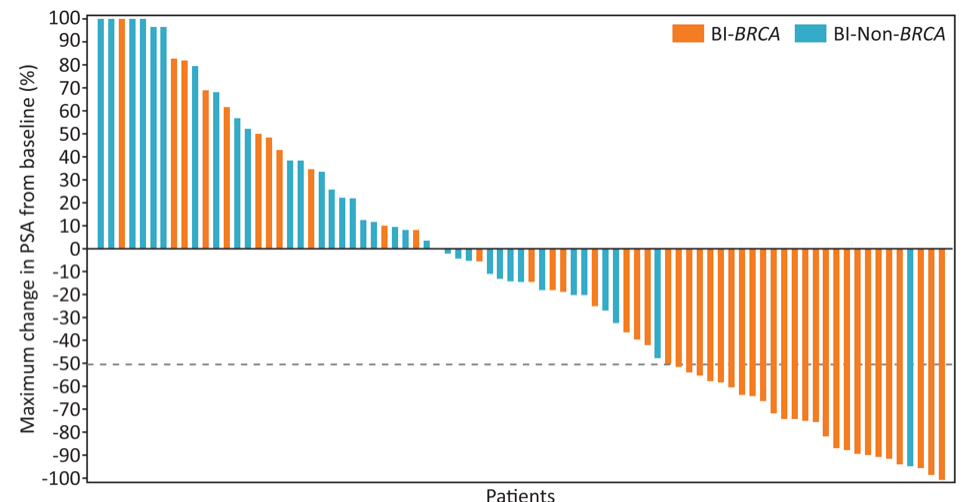
66% of patients with biallelic BRCA had a duration of treatment ≥6 months. Grade 3/4 treatment-emergent adverse events were mostly hematologic—anemia (29%), thrombocytopenia (15%) and neutropenia (7%)—and managed with dose interruption or modification.
Dr. Smith concluded the preplanned interim analysis of GALAHAD with several concluding points:
- Niraparib has high clinical activity in patients with biallelic BRCA mutations with an objective response rate of 41%
- The composite response rate for patients with measurable disease was 66% and for patients with non-measurable disease was 59%
- Median rPFS for BRCA patients was 8.2 months
- The efficacy and safety of niraparib in patients with mCRPC and DNA repair defects will continue to be evaluated in ongoing clinical trials, including GALAHAD, MAGNITUDE, and QUEST
Clinical Trial Identification: NCT02854436.
Presented by: Matthew R. Smith, MD, PhD, Director of the Genitourinary Malignancies Program, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts
Co-Authors: S. Sandhu1, W. Kelly2, H. Scher3, E. Efstathiou4, P. Lara5, E. Yu6, D. George7, K. Chi8, F. Saad9, J. Summa10, J. Freedman10, G. Mason11, E. Zhu12, D. Ricci13, J. Simon14, S. Cheng15, K. Fizazi16
1. Peter MacCallum Cancer Centre, Melbourne, AU
2. Kimmel Cancer Center-Thomas Jefferson University, Philadelphia, US
3. Memorial Sloan-Kettering Cancer Center, New York,
4. The M. D. Anderson Cancer Center, Houston, US
5. University of California Davis Cancer Center, Sacramento, US
6. University of Washington Seattle Cancer Care Alliance, Seattle, US
7. Duke University Medical Center, Durham, US
8. BC Cancer Agency – Vancouver, Vancouver, CA
9. Hospital St. Luc du CHUM, Montreal, CA
10. Janssen Research & Development, Los Angeles, U
11. Janssen Research & Development, Spring House, US
12. Janssen Research & Development, Raritan, US
13. Janssen Research and Development, Spring House, US
14. Janssen R&D/Johnson & Johnson Pharmaceutical R&D, Spring House, US
15. Bristol-Myers Squibb, Princeton, US
16. Institut Gustave Roussy, Villejuif, FR
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct, 2019 in Barcelona, Spain
Reference:
