Barcelona, Spain (UroToday.com) The SORCE trial is a randomized placebo-controlled trial of adjuvant sorafenib for resected high and intermediate risk (Leibovich score 3-11) renal cell carcinoma. Between July 2007 and April 2013 1,711 patients were randomized 2:3:3 to receive 3 years of 3 years of placebo, 1 year of sorafenib (400mg twice daily) followed by 2 years of placebo, or 3 years of sorafenib. The primary endpoints were disease-free survival and overall survival. Of note, in October, 2009, due to a high frequency of study withdrawal due to toxicity, the sorafenib dosing schedule was changed to 400mg once daily, with the ability to titrate up to twice daily or down to every other day pending tolerability. 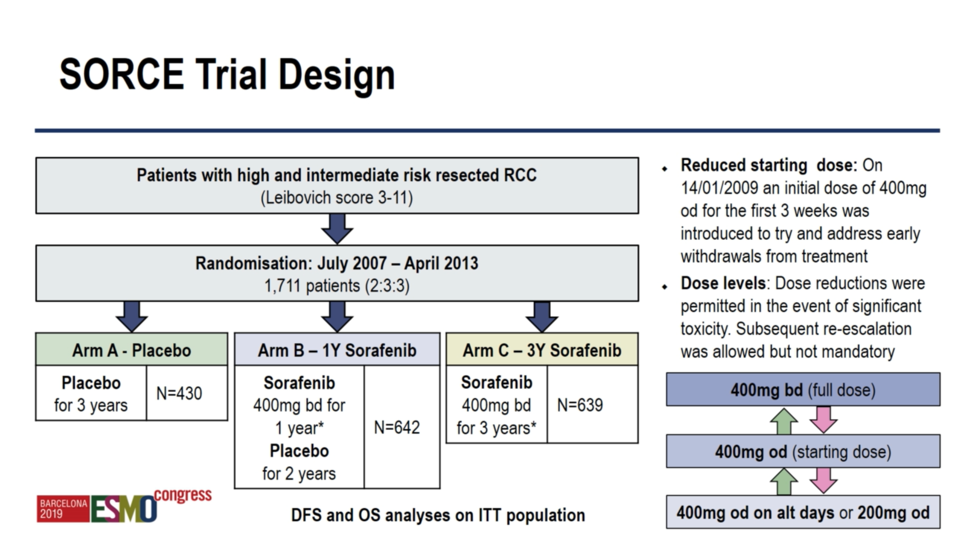
At baseline, patients were fairly well-matched across study arms and representative of a typical adjuvant renal cell carcinoma patient population. Mean age was 58, and clear cell was the most common histology (83%-86%) followed by papillary (6%-9%). With respect to risk score, a slight majority were considered intermediate risk (52%-54%) versus high risk (46%-48%). 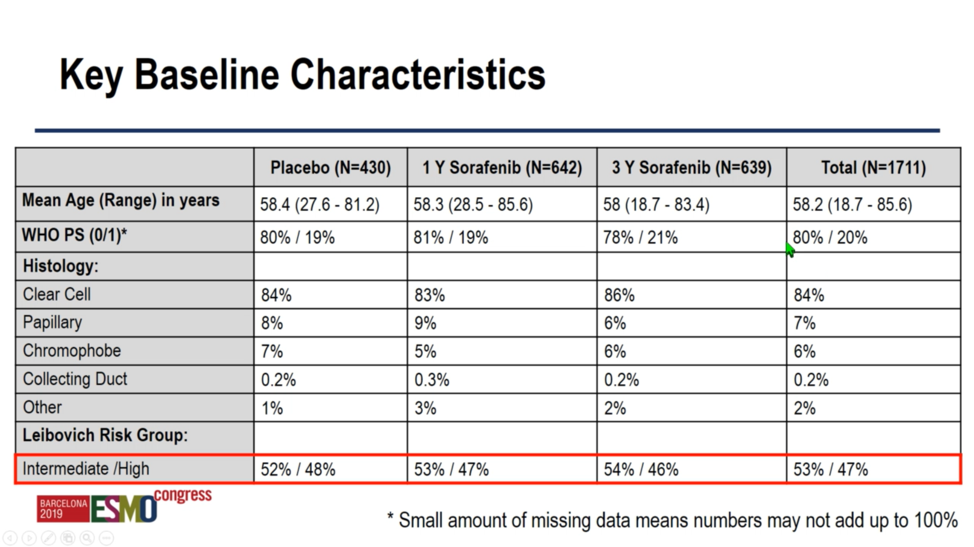
Given the results of ASSURE and S-TRAC, investigators in 2017 shifted the focus of SORCE to the analysis of 3 years of sorafenib versus placebo. With respect to tis primary analysis, investigators repost no significant difference between sorafenib and placebo with respect to 5 year disease free survival 67% versus 65%) or 10 year disease free survival (54% versus 53%). Analysis by hazard ratio (1.01, confidence interval 0.82 – 1.23) or by three-year restricted mean survival time difference 0.01 (confidence interval -0.49 – 0.48) yielded similarly indistinguishable results. 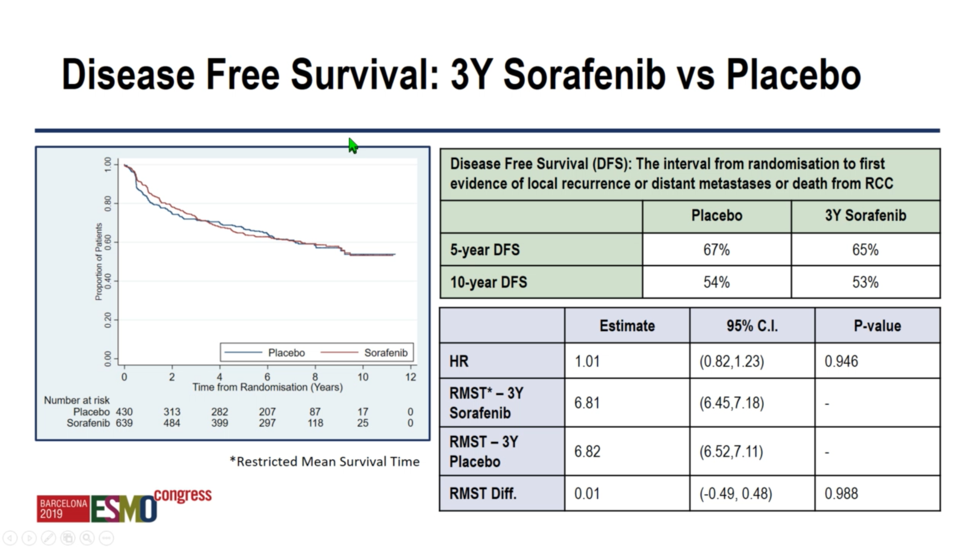
Regarding overall survival, investigators again found no difference between sorafenib and placebo – either 1 year or 3 years. Encouragingly, patients generally achieved good clinical outcomes, with 10-year overall survival of 69%-70% irrespective of treatment arm. 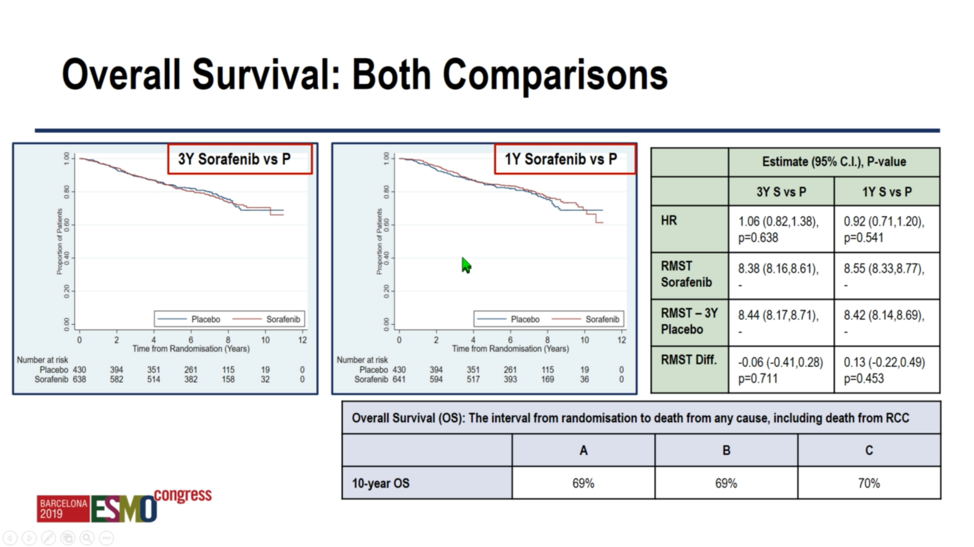
Sorafenib was difficult to tolerate, particularly given the adjuvant nature of the study. Whereas 55.1% of patients in the placebo arm were able to complete treatment – 24.2% stopped for disease progression – only 33% and 25.4% were able to complete a 1-year or 3-year course of sorafenib respectively, owing most commonly to excessive toxicity, but also to disease recurrence or patient refusal. 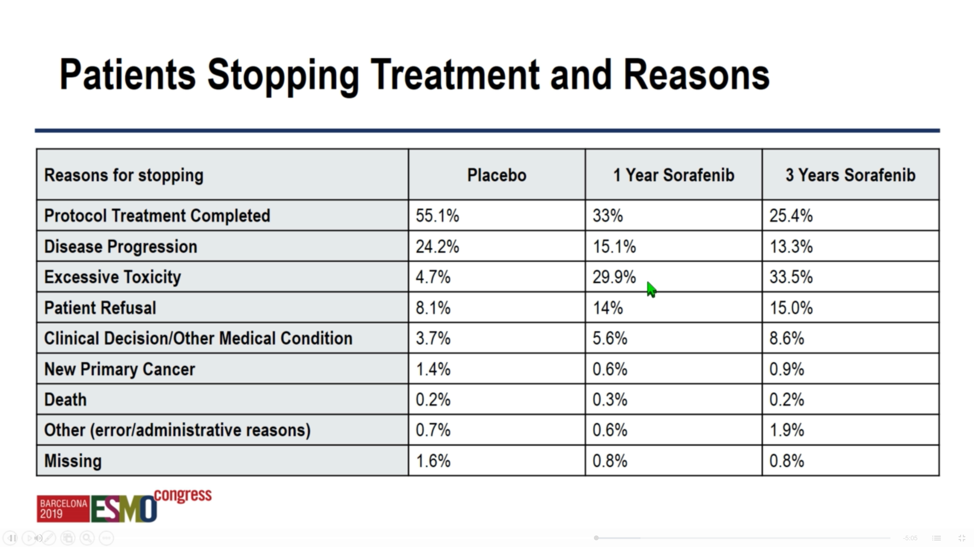
With regard to toxicities, hypertension was the most common grade 3 toxicity, affecting 24%-26% of the sorafenib arms. Additionally, approximately 70% of patients in the active treatment arms experienced rash, and 77%-79% experienced hand-foot syndrome (including approximately 25% grade 3 in each arm). Of note, a remarkably high proportion (20%) of patients in the placebo arm developed grade 3 hypertension as well. 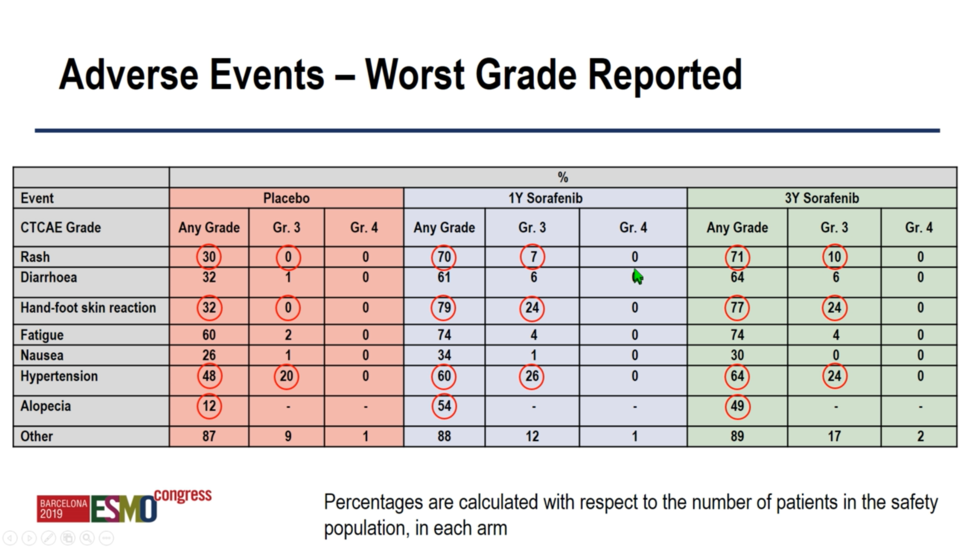
In conclusion, sorafenib monotherapy has no role in the adjuvant treatment of intermediate-high risk resected renal cell carcinoma. Such patients should continue to receive active surveillance as standard of care. All major adjuvant trials in renal cell carcinoma to date have utilized small molecule inhibitors or anti-angiogenic agents; future work will investigate the efficacy of perioperative checkpoint blockade immunotherapy in this setting.
Presented by: Tim Eisen, PhD, University of Cambridge, Cambridgeshire, UK
Written by: Michael Lattanzi, MD, Medical Oncology Fellow, Memorial Sloan Kettering Cancer Center, Twitter: @MikeLattanzi at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct, 2019 in Barcelona, Spain
