Barcelona, Spain (UroToday.com) Immune-checkpoint inhibitor therapy has yielded notable clinical benefits for patients with metastatic renal cell carcinoma (mRCC). 1,2 ADATPeR is the first prospective study evaluating the role of anti-PD1 agents in the neoadjuvant setting prior to cytoreductive nephrectomy in treatment-naïve patients with metastatic clear cell renal cell carcinoma. At ESMO 2019 clinical congress, Dr. Au presented results of their multiomic analyses to resolve spatial heterogeneity and temporal dynamics in putative biomarkers of response to anti-PD1 blockade.
ADATPeR is a single center study whereby patients receive nivolumab (3mg/kg every 2 weeks) pre- and post-operatively until progressive disease. Patients deemed clinically suitable for nephrectomy at baseline were scheduled for surgery after the fourth cycle of treatment. Patients not clinically suitable for nephrectomy at baseline would undergo surgery if an excellent clinical response was observed. The primary endpoint was safety, and secondary endpoints were response evaluation and exploratory biomarker analysis. Multiregion tumour biopsies were obtained at baseline, on-treatment (week 9) and at progressive disease. Whole-exome sequencing was performed to infer somatic mutations and predict candidate neoantigens. Tumour immune microenvironment was evaluated using a RNA-seq-derived immune signature and by stromal and intraepithelial tumour infiltrating lymphocytes assessments.
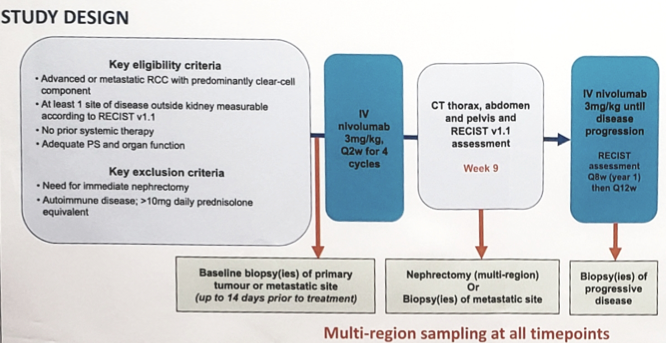
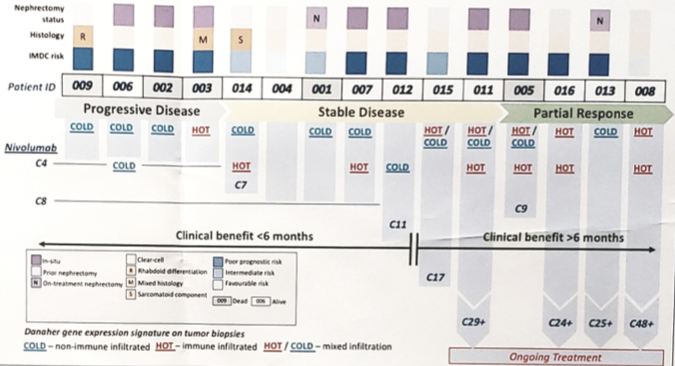
From October 2015 to June 2018, 16 patients were enrolled and 15 patients received treatment. The median age was 56 years (range 49-80), 87% were male, 40% were IMDC poor risk, and 100% were clear cell histology. At median follow-up of 12.5 months, nivolumab had an acceptable side-effect profile. Five patients continued on treatment, and the median treatment cycles was 7 (range 4-29), with an overall response rate of 27%. Five deaths had occurred and 10 progression events were observed, with a median OS of 17.5 months and median PFS of 4.1 months. Among the 15 patients, 9 patients had the primary tumor in situ (two patients underwent a nephrectomy during nivolumab treatment), with four patients showing primary tumor shrinkage at cycle 4. Preliminary transcriptome analyses of pre-treatment biopsies (33 samples from 14 patients; up to 4 regions per case) revealed enrichment for primary-resistance (defined as PD within 2m; n = 4) with immune ‘cold’ tumours, distinct from ’hot’ tumours. Histologic tumour infiltrating lymphocytes scoring showed concordant immune phenotypic clusters. Primary-resistant cases demonstrated 0% on-treatment stromal- and IE- tumour infiltrating lymphocytes. In contrast, the investigators observed heavy on-treatment stromal tumour infiltrating lymphocytes (70-90%) and intraepithelial tumour infiltrating lymphocytes (30-90%) across 7 regions at nephrectomy in an exceptional responder receiving ongoing treatment (>24 cycles).
Dr. Au concluded the ADATPeR study with several take home messages:
- This is the first neoadjuvant immune checkpoint inhibitor study pre-cytoreductive nephrectomy, incorporating multiomic analyses of putative biomarkers
- Baseline immune gene expression signature is distinct in responders compared with non-responders
- On-treatment intraepithelial tumour infiltrating lymphocytes were prominent in those deriving durable clinical benefit
- Analyses for T-cell repertoire dynamics and immune infiltration patterns are ongoing, as well as genomic analyses (mutations and neoantigen landscape)
Clinical Trial Identification NCT02446860
Presented by: Lewis Au, Royal Marsden Hospital NHS Foundation Trust, London, UK
Co-Authors: L. Au 1, K. Litchfield 2, A. Rowan 2, S. Horswell 2, F. Byrne 2, D. Nicol 1, N. Fotiadis 3, R. Salgado 4, S. Hazell 1, J.I. Lopez 5, E. Hatipoglu 6, L. Del Rosario 1, L. Pickering 1, M. Gore 1, B. Chain 7, S. Quezada 8, J. Larkin 1, C. Swanton 2, S. Turajlic 2
1. Royal Marsden Hospital NHS Foundation Trust, London, UK
2. The Francis Crick Institute, London, UK
3. Royal Marsden NHS Foundation Trust, Sutton, UK
4. St-Augustinus Ziekenhuis, Wilrijk, BE
5. Cruces University Hospital, Bilbao, ES
6. University College London Cancer Institute, London, UK
7. Imperial College London, London, UK
8. University College London, London, UK
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct 2019 in Barcelona, Spain
References:
