Barcelona, Spain (UroToday.com) Refractory disease represents a critical issue in germ cell tumor (GCT) management with < 5% of patients surviving. Many chemotherapeutic and targeted treatment strategies have been evaluated with low response rates highlighting an urgent need for new treatment options in this clinical setting. APACHE (NCT03081923) is an open label, phase 2 study of Durvalumab (Durva) alone or in combination with Temelimumab (Treme) in patients with refractory germ cell tumors (GCTs). The previously published first interim results demonstrated futility in the Durva monotherapy arm.1 This abstract presents updated results from an expanded Durva + Treme combination therapy cohort.
The APACHE study enrolled patients who had failed two prior chemotherapy regimens for metastatic GCT. Patients on Arm A received Durva monotherapy and patients on Arm B received Durva + with Treme for 4 cycles, followed by Durva alone. The primary study endpoint is the modified objective response-rate (ORR) as measured by RECIST 1.1 complete or partial response (PR) or stable disease (SD) with a reduction of serum tumor markers (STMs) of greater than 10%.
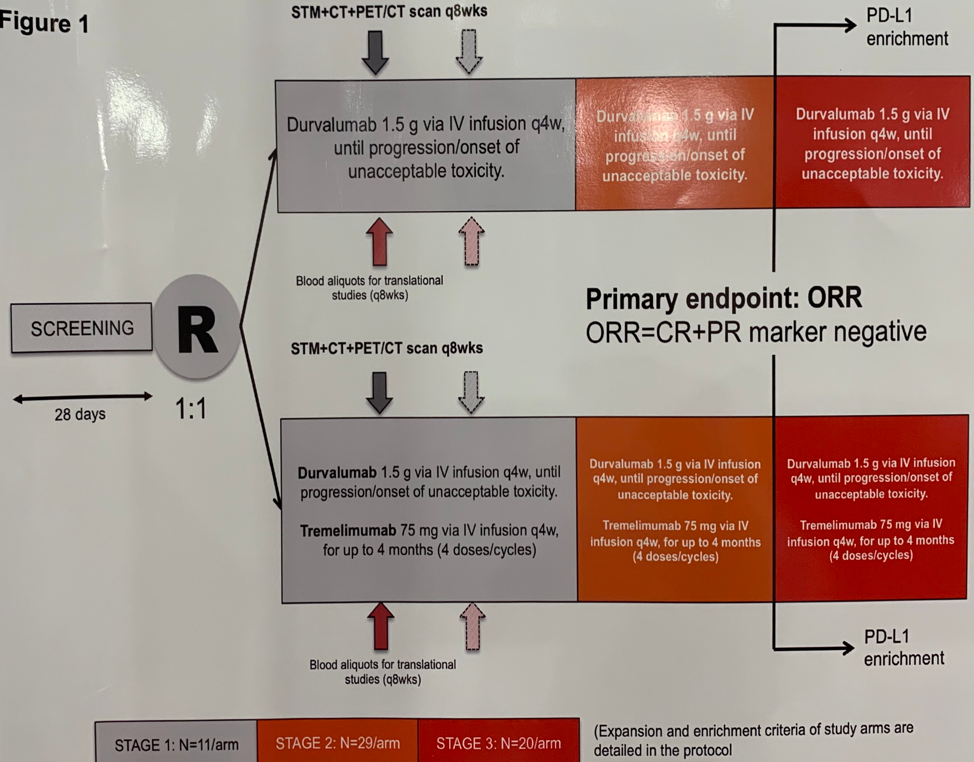
At the time of data cutoff, 31 patients were enrolled in the study, 11 to arm A and 20 to arm B. The median age of patients was 35; 24 had gonadal and 20 had extragonadal GCTs. 3 patients had seminoma and the remaining 28 had non-seminomatous GCTs. The median tumor mutational burden (TMB) in the study cohort was 4 mutations per megabase. Only 1 patient showed microsatellite instability who was treated on Arm A. In the expanded cohort treated on Arm B with combination Durva + Treme therapy, there were two responses, resulting in an ORR of 11%. One patient with a seminoma experienced a PR and one patient had SD with reduction in STMs. Two additional patients had stable disease. There was no correlation of PD-L1 or TMB with response to Durva + Treme.
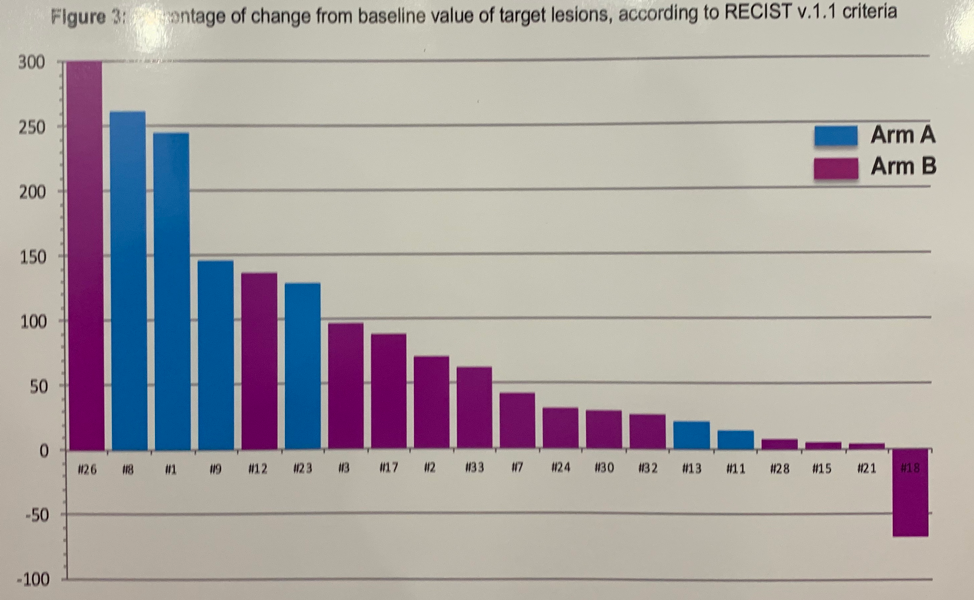
The median progression-free survival (PFS) with Durva monotherapy was 1.25 months and median overall survival (OS) was 3.36 months. No patients receiving Durva alone reached 6 months progression-free. The median PFS among patients receiving Durva + Treme was 1.98 months with a median OS of 5.25 months. 10% of patients were progression-free at 6 months and 25.5% were still alive at 13 months.
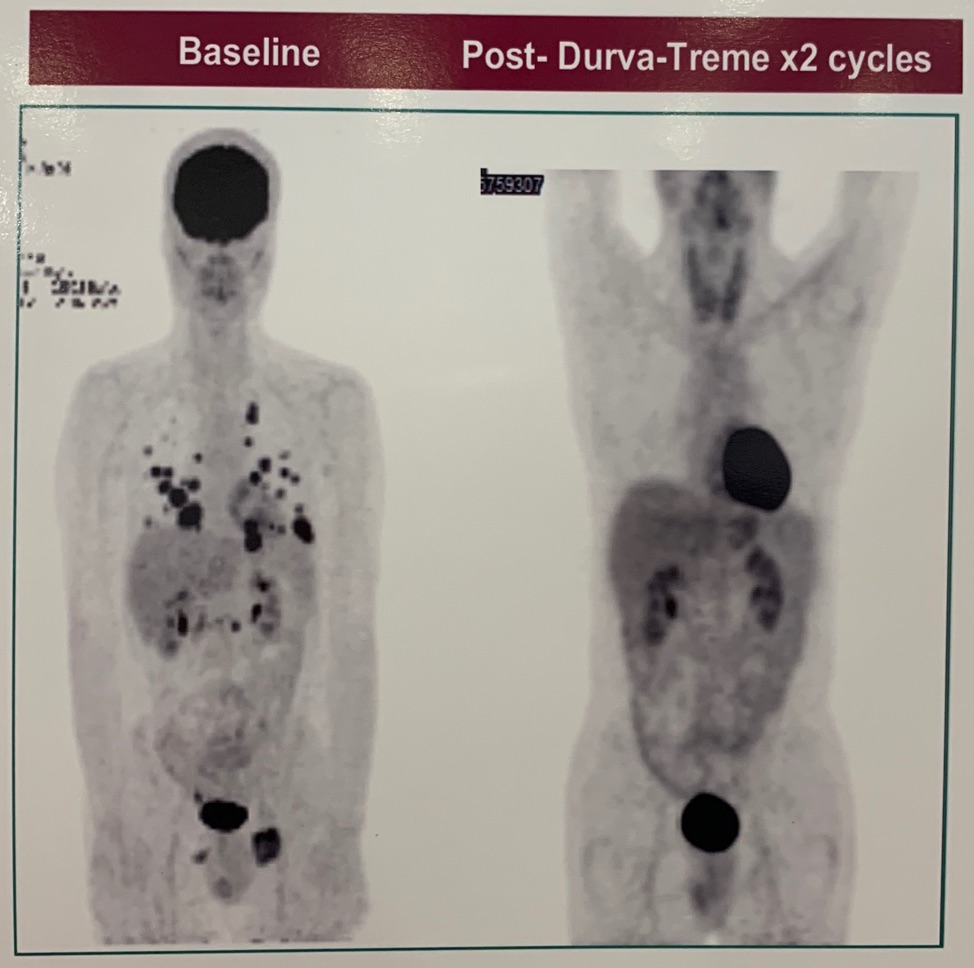
One patient with pure seminoma had an outstanding response to Durva + Treme. After two cycles of therapy there was a significant reduction in tumor burden. Tumor analysis revealed PD-L1 negative, microsatellite stable, and low TMB.
In conclusion, the APACHE study found that patients with refractory GCT do not respond to Durvalumab monotherapy while Durvalumab plus Temelimumab has limited activity. The combination therapy resulted in few objective responses, though a small percent of patients were alive after more than a year of starting treatment suggesting a possible long-term benefit in select patients. Conventional biomarkers did not allow for patient selection, thus further research is needed to identify patients who will benefit from combination immunotherapy.
Clinical trial information: NCT03081923
Presented by: Elena Faré, MD, Medical Oncologist, Istituto Nazionale dei Tumori di Milano – Fondazione IRCCS, Milan, Italy
Written by: Jacob Berchuck, MD, Medical Oncology Fellow at the Dana-Farber Cancer Institute, Twitter: @jberchuck, at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct 2019 in Barcelona, Spain
References:
- Necchi A, Giannatempo P, Raggi D, et al. An Open-label Randomized Phase 2 study of Durvalumab Alone or in Combination with Tremelimumab in Patients with Advanced Germ Cell Tumors (APACHE): Results from the First Planned Interim Analysis. Eur Urol. 2019 Jan;75(1):201-203.
