Barcelona, Spain (UroToday.com) Relative to other solid tumors, prostate cancer is viewed as an immunologically “cold” tumor with less robust responses to immunotherapy than other diseases such as melanoma or lung cancer. Early data from trials of immunotherapy in metastatic castration resistant prostate cancer (mCRPC) do however suggest that a small percentage of patients respond to this approach and may have durable benefit. A recent analysis has suggested that mCRPC patients with deficient DNA mismatch repair or high levels of DNA microsatellite instability may uniquely benefit from immunotherapy.1 Many research efforts are now focused on ways to augment immunotherapy response in mCRPC, including through combination chemotherapy and immunotherapy regimens.
In an ESMO 2019 poster, Karim Fizazi, MD, PhD, and colleagues report interim analysis of the nivolumab (anti-PD1) plus docetaxel treatment arm from the CheckMate 9KD trial (NCT03338790). This represents Arm B of the trial, which includes chemotherapy naïve mCRPC patients with pre-assessed homologous recombination deficiency (HRD) status. Patients were treated with up to 10 cycles of combination 360 mg nivolumab every three weeks and 75 mg/m2 docetaxel every three weeks, then switched to 480 mg nivolumab every 4 weeks for up to two years up until progression or intolerance. Primary endpoints are overall response rate and prostate specific antigen (PSA) response rate. Secondary endpoints include radiographic progression free survival (PFS) and safety. Exploratory correlative studies included association with response and HRD as well as with tumor mutational burden (TMB).
The overall response rate for the 19 patients assessed with measurable disease was 37% with one complete response and six partial responses. The PSA response rate was 46.3%. The median radiographic PFS was 8.2 months, with 71.5% of patients free of radiographic progression at six months of follow-up. 93% of patients reported treatment-related adverse effects, with 49% at the grade 3/4 level. The most common grade 3/4 adverse events were neutropenia (29.3%), febrile neutropenia (9.8%), diarrhea (7.3%) and asthenia (7.3%).
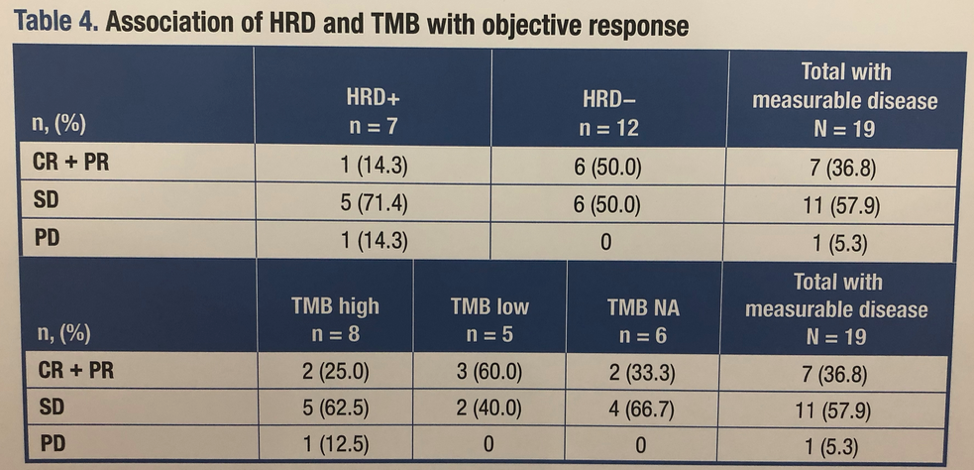
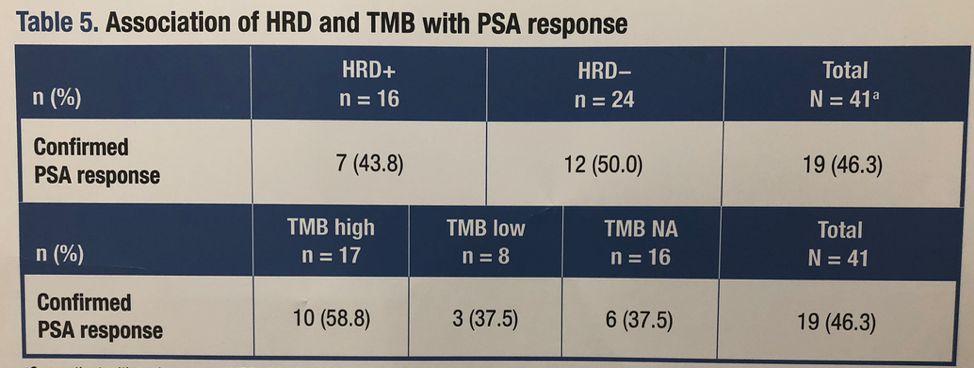
The median tumor mutational burden in the overall trial is 3.51 Mut/Mb. Of patients with measurable disease, seven were positive for a mutation associated with HRD (HRD+), 12 were not. Of all patients, 16 were HRD+, 24 were not. While only one HRD+ patient had either a complete or partial response by imaging where as six lacking HRD had such a response, this comparison is very limited due to small sample size. Overall, there was no clear association between overall response rate or PSA response rate and either TMB or HRD status. These data are summarized in Table 4 above and Table 5 below.
While future plans are being developed for larger trials with this combination, given the history of negative docetaxel combination trials in mCRPC, a biomarker-based approach may be more likely to identify patients that respond to therapy.
Presented by: Karim Fizazi, MD, PhD, Medical Oncologist, Head of the Department of Cancer Medicine at the Institut Gustave Roussy, Veillejuif, France
Written by: Alok Tewari, MD, PhD, Medical Oncology Fellow at the Dana-Farber Cancer Institute, at the 2019 European Society for Medical Oncology Congress (#ESMO19), September 27th-October 1st, 2019, Barcelona, Spain
References:
