Barcelona, Spain (UroToday.com) On the basis of noninferiority outcomes for patients with metastatic disease who received sunitinib versus patients who underwent upfront cytoreductive nephrectomy followed by sunitinib,1 the role of upfront cytoreductive nephrectomy in patients with metastatic renal cell carcinoma (RCC) who require systemic therapy has been revised. However, the activity of new combination treatments on the primary tumour is unknown. The JAVELIN Renal 101 trial demonstrated longer progression-free survival (median 13.8 vs 8.4 months; HR 0.69; p < 0.001) and higher objective response rate (51% vs 26%) with avelumab + axitinib versus sunitinib in patients with advanced RCC.2 Furthermore, benefit was observed in all risk subgroups and in patients irrespective of prior cytoreductive nephrectomy. These results led to the US FDA’s approval of the combination therapy in the first line setting for advanced RCC. Since the potential role of new combination treatments to reduce the primary tumor burden has not yet been reported, Dr. Albiges and colleagues presented results of their post hoc analysis of patients enrolled in JAVELIN Renal 101 who had not undergone upfront cytoreductive nephrectomy and had renal target lesions.
There were 886 eligible patients with clear cell advanced RCC, no prior systemic therapy for advanced RCC, and ECOG performance status 0-1 that were randomized 1:1 to receive either avelumab + axitinib (n = 442) or sunitinib (n = 444) following a standard dose and schedule. This was a post hoc analysis to investigate the extent of primary renal tumour shrinkage in 179 patients (20.2%; 90 in the avelumab + axitinib arm; 89 in the sunitinib arm) with renal target lesions who did not undergo upfront cytoreductive nephrectomy.
Among the 179, 117 patients had renal target lesions at baseline: 55/90 on the avelumab + axitinib arm and 62/89 in the sunitinib arm. Baseline characteristics were balanced between the two arms in this subgroup, including 0.9% with favorable risk disease, 59.8% with intermediate risk disease, and 39.3% with poor IMDC risk disease. Of the patients with renal target lesions who did not undergo upfront cytoreductive nephrectomy in the avelumab + axitinib vs sunitinib arms, 34.5% vs 9.7% had ≥30% shrinkage for best percent change in renal target lesions from baseline.
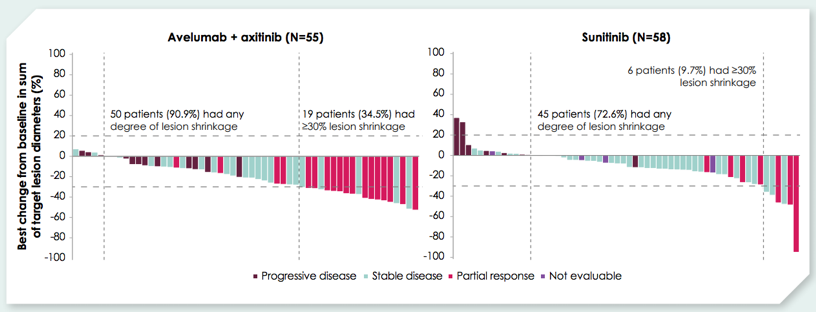
The median time to ≥ 30% shrinkage in renal target lesions was 4.4 months for avelumab + axitinib vs 7.1 months for sunitinib. Patients in the combination arm had 3.7 months longer mean duration of ≥ 30% renal target lesion shrinkage than those on the sunitinib arm: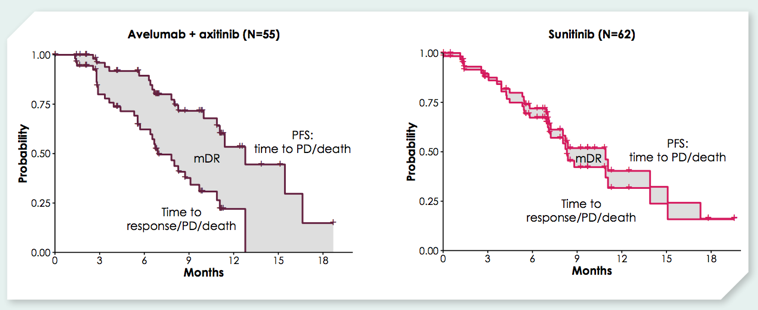
Compared with patients in the overall trial population who had a nephrectomy, patients who did not undergo an upfront cytoreductive nephrectomy had reduced PD-L1 expression, comparable molecularly heterogeneous disease, based on the subtype-specific mRNA signatures with no subtype being dominant. Neither PD-L1 status nor tumor mutational burden differentiated PFS in either arm. Higher CD8A expression favored the combination arm, whereas high KDR or PTGER3 expression favored the sunitinib arm. The IMotion150 T effector gene signature was associated with longer PFS in the combination arm, whereas the JAVELIN Renal 101 gene signature did not differentiate PFS in either arm.
Dr. Albiges provided the following concluding remarks to this post-hoc analysis of JAVELIN Renal 101:
- Avelumab + axitinib resulted in greater shrinkage of the primary renal tumour versus sunitinib in patients with advanced RCC who did not undergo upfront cytoreductive nephrectomy
- This is the first report of the efficacy of immuno-oncology + tyrosine kinase inhibitor therapy on the primary tumour in the context of metastatic RCC and provides insight into future neoadjuvant strategies in advanced RCC
- An open-label, single arm, phase II trial is investigating avelumab + axitinib as neoadjuvant therapy in patients with localized RCC
Clinical trial identification NCT02684006
Presented by: Laurence Albiges, Institut Gustave Roussy, Villejuif, FR
Co-Authors: B. Rini 2, J. Haanen 3, R. Motzer 4, C. Kollmannsberger 5, S. Negrier 6, F. Nole 7, J. Bedke 8, M. Bilen 9, P. Nathan 10, Y. Tomita 11, B. Huang 12, K. Ching 13, A. Chudnovsky 14, P. Robbins 15, A. Di Pietro 16, D. Thomaidou 17, T. Choueiri 18
2. Cleveland Clinic, Cleveland, US
3. Netherlands Cancer Institute/Antoni van Leeuwenhoek hospital (NKI-AVL), Amsterdam, NL
4. Memorial Sloan-Kettering Cancer Center, New York, US
5. BC Cancer Agency – Vancouver, Vancouver, CA
6. Centre Léon Bérard, Lyon, FR
7. Istituto Europeo di Oncologia, Milan, IT
8. University of Tuebingen, Tuebingen, DE
9. Emory University Winship Cancer Institute, Atlanta, US
10. Mount Vernon Cancer Centre and East & North Herts, NHS Trust, London, UK
11. Yamagata University Hospital, Yamagata, JP
12. Pfizer Inc., Groton, US
13. Pfizer Inc., San Diego, US
14. Pfizer Inc., Cambridge, US
15. Pfizer Inc – USA, New London, US
16. Pfizer Inc., Milano, IT
17. Pfizer Hellas, Athens, GR
18. Dana-Farber Cancer Institute, Boston, US
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept – 1 Oct 2019 in Barcelona, Spain
References
- Mejean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal cell carcinoma. N Engl J Med 2018 Aug 2;379(5):417-427.
- Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380(12):1103-1115.
