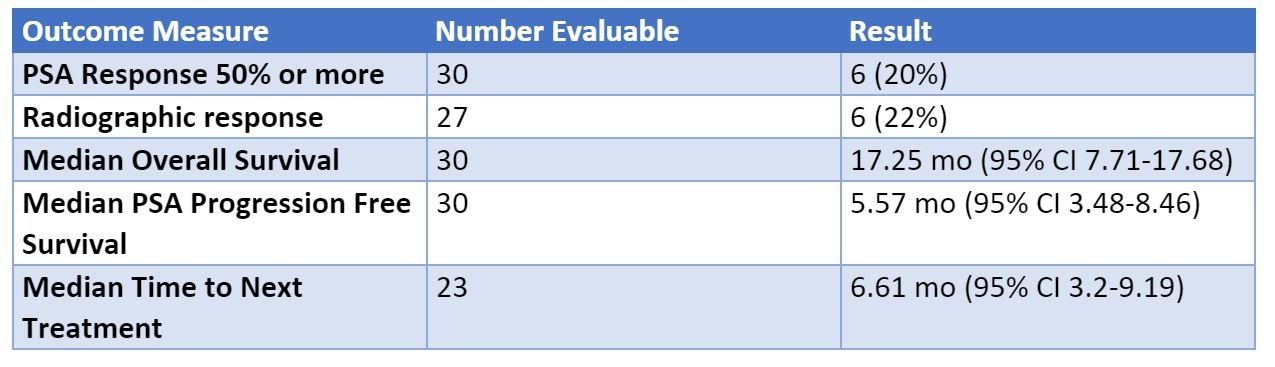
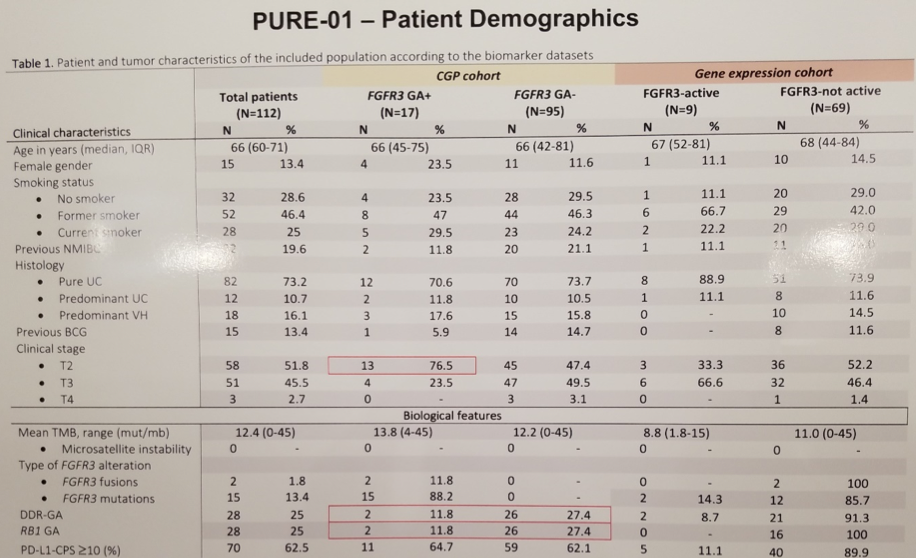
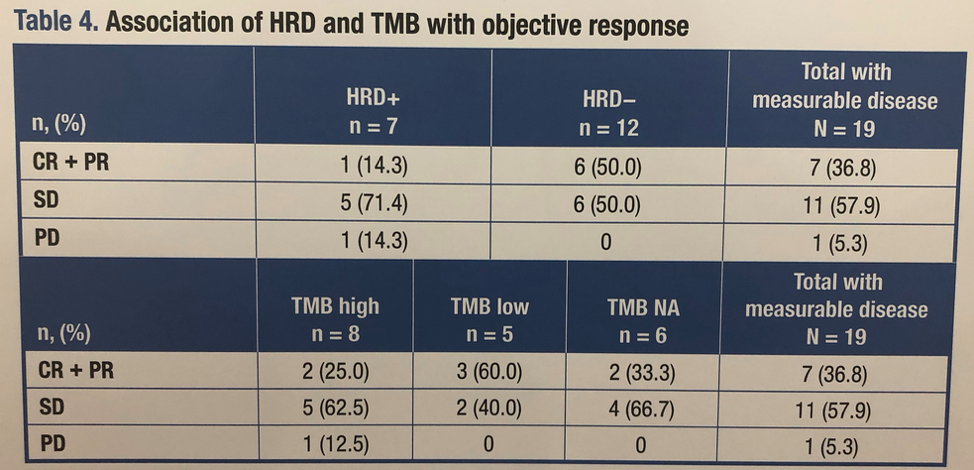
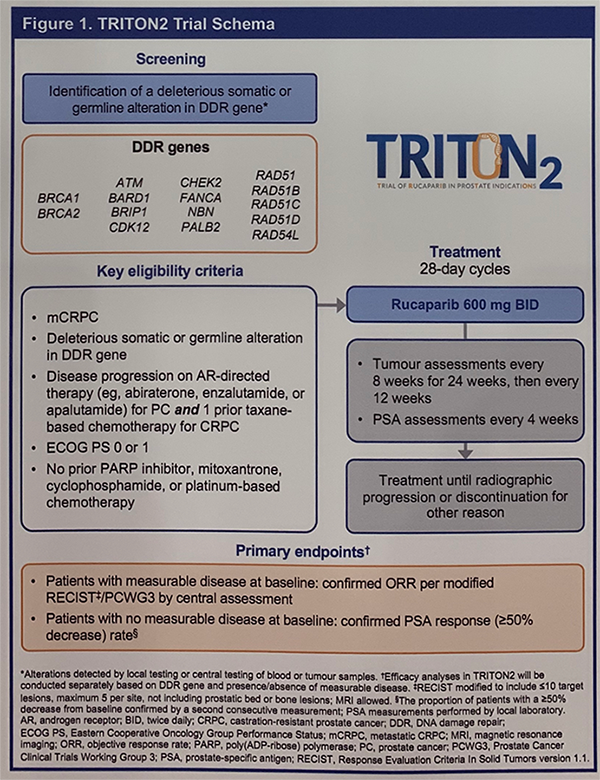
ESMO 2019: Pembrolizumab with Enzalutamide in mCRPC: Examination of Tumor Infiltrating Immune Cells and Fecal Microbiota, A Phase II Study – A Medical Oncologist’s Perspective
Barcelona, Spain (UroToday.com) Relative to other solid tumors, prostate cancer is viewed as an immunologically “cold” tumor with less robust responses to immunotherapy than other malignancies. Early data from trials of immunotherapy in metastatic castration-resistant prostate cancer (mCRPC) do however suggest that a small percentage of patients respond to this approach and may have durable benefit. […]