ESMO 2019: An Adaptive, Biomarker-Directed Platform Study with Durvalumab in Combination with Targeted Therapies in Metastatic Urothelial Cancer (BISCAY)
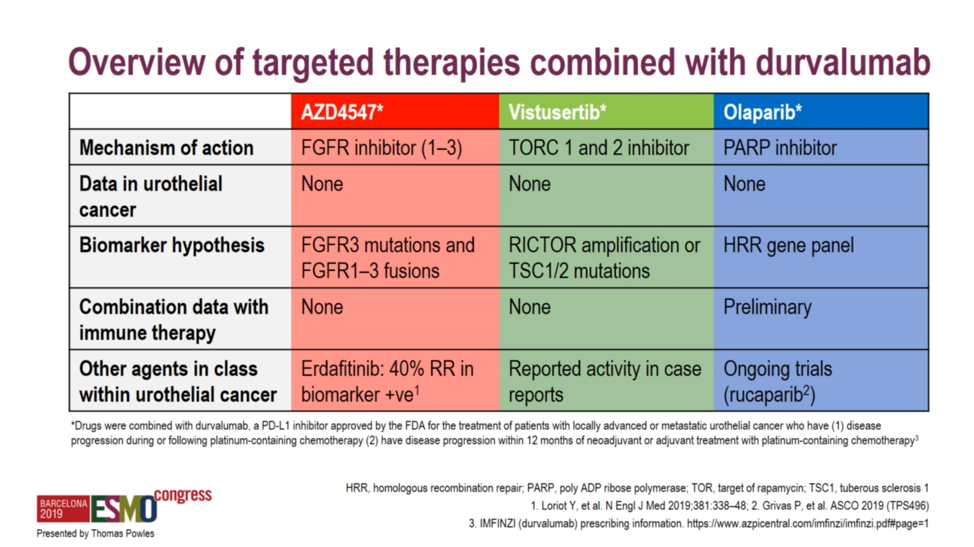
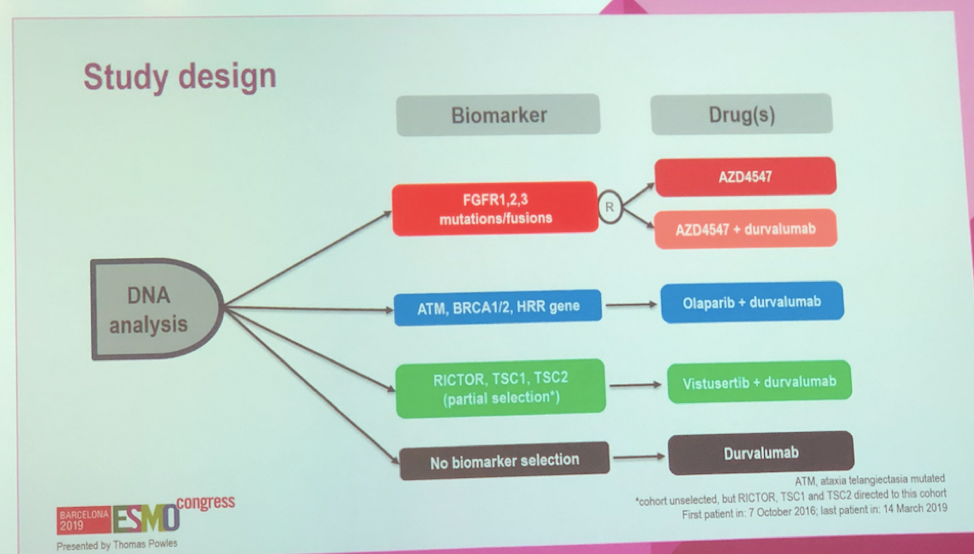
Barcelona, Spain (UroToday.com) BISCAY was a novel biomarker-directed platform for testing multiple immune-oncology combinations in metastatic urothelial carcinoma (mUC). Specifically, BISCAY sought to evaluate an anti-PD-L1 backbone in combination with any of three specific biomarker-directed targeted therapies: FGFR inhibition with AZD4547, TORC1/2 inhibition with vistusertib, and PARP inhibition with olaparib. Patients were assigned to treatment […]