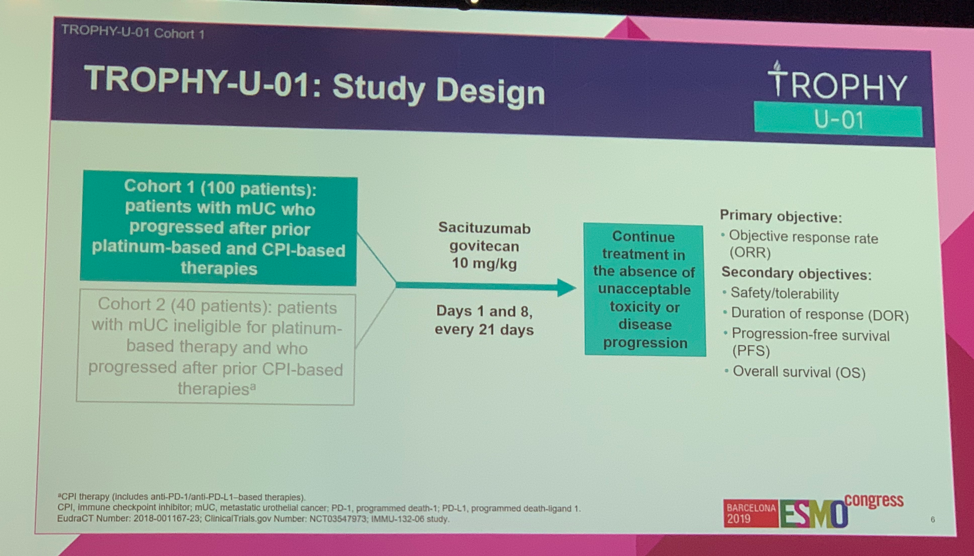
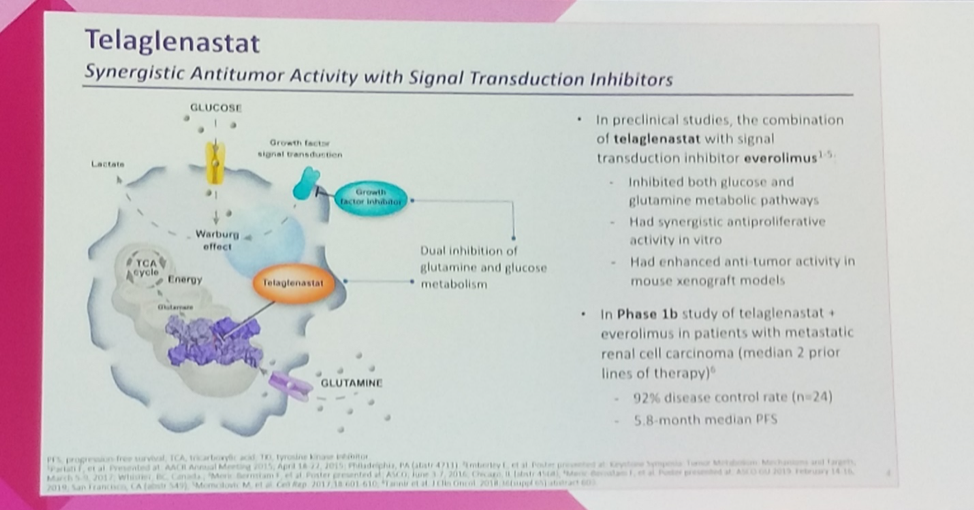
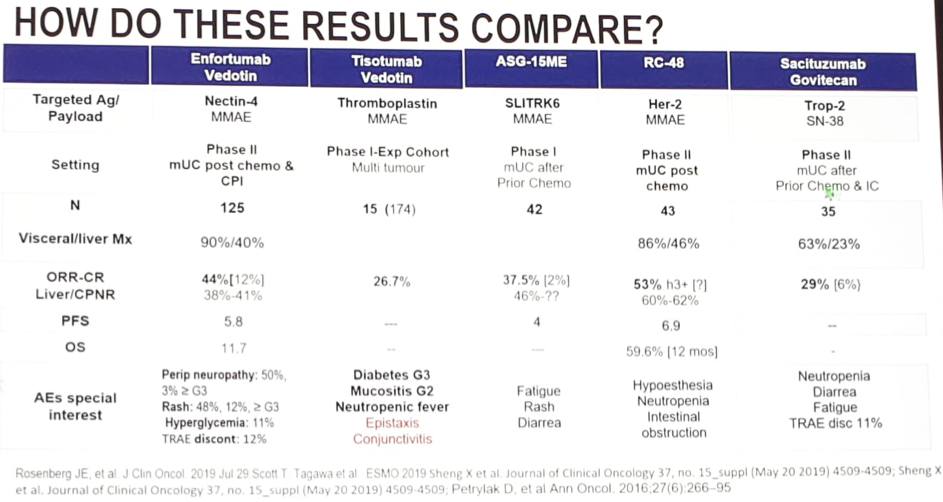
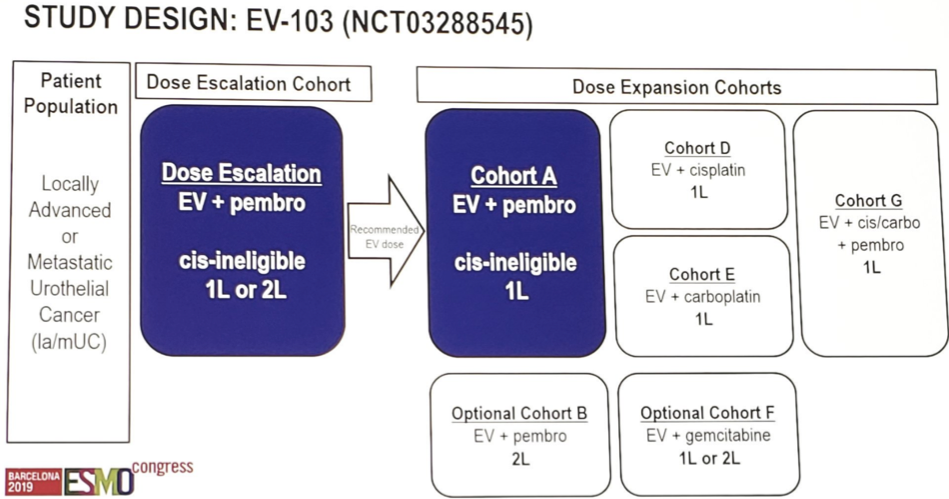
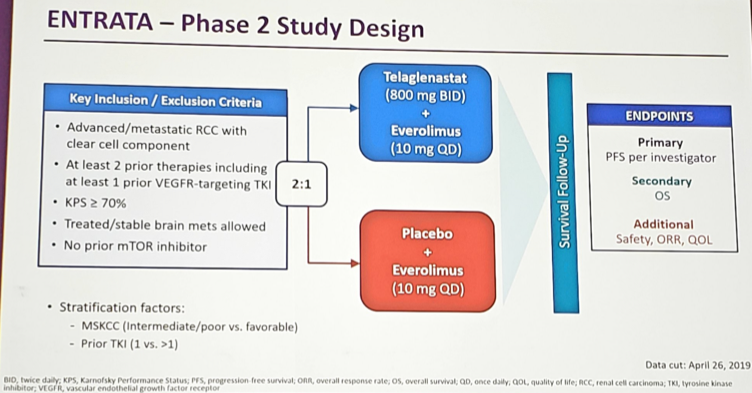
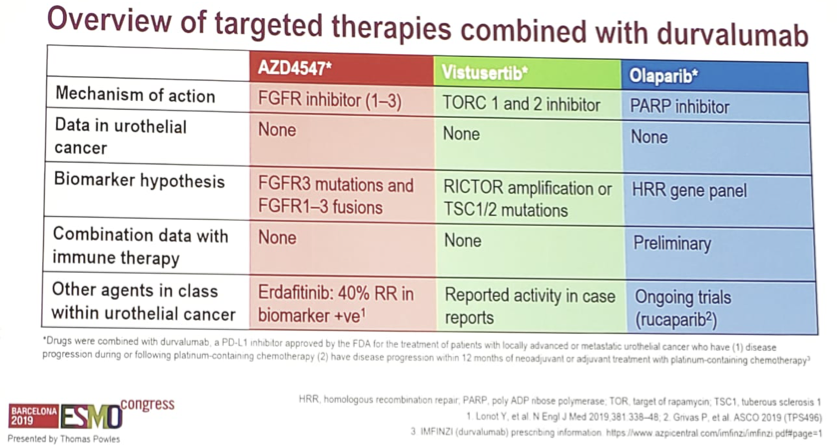
ESMO 2019: Initial Results of EV-103, A phase 1b Study Evaluating the Safety and Activity of Enfortumab Vedotin Plus Pembrolizumab – A Medical Oncologist’s Perspective
Barcelona, Spain (UroToday.com) Platinum-based chemotherapy is the standard of care in patients with locally advanced or metastatic urothelial carcinoma, but this therapy is often toxic with overall response rates of 50% or less. These tumors highly and consistently express the type I transmembrane cellular adhesion protein, Nectin-4. Enfortumab vedotin (EV) is an antibody-drug conjugate against […]